Chapter 23
Organic Chemistry
By Boundless
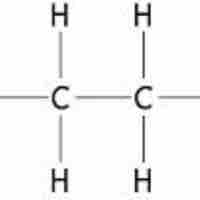
Alkanes are relatively unreactive hydrocarbons that contain no double or triple bonds in their carbon skeletons.

Alkanes are generally unreactive, but can participate in oxidation, halogenation, and cracking reactions.
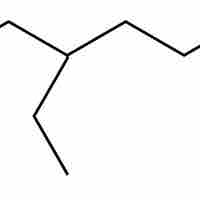
Hydrocarbon structures can be drawn from the IUPAC names of chemical compounds by starting with the carbon backbone and adding substituents.
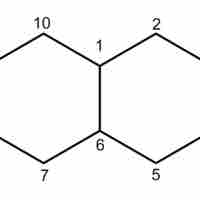
Cycloalkanes are saturated hydrocarbons that contain a ring in their carbon backbones.
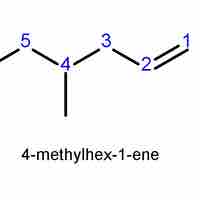
Alkenes and alkynes are named similarly to alkanes, based on the longest chain that contains the double or triple bond.
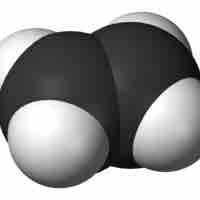
Due to the presence of a double bond in their carbon skeletons, alkenes are more reactive than their related alkanes.
.jpg)
Alkenes and alkynes are more reactive than alkanes due to their pi bonds.
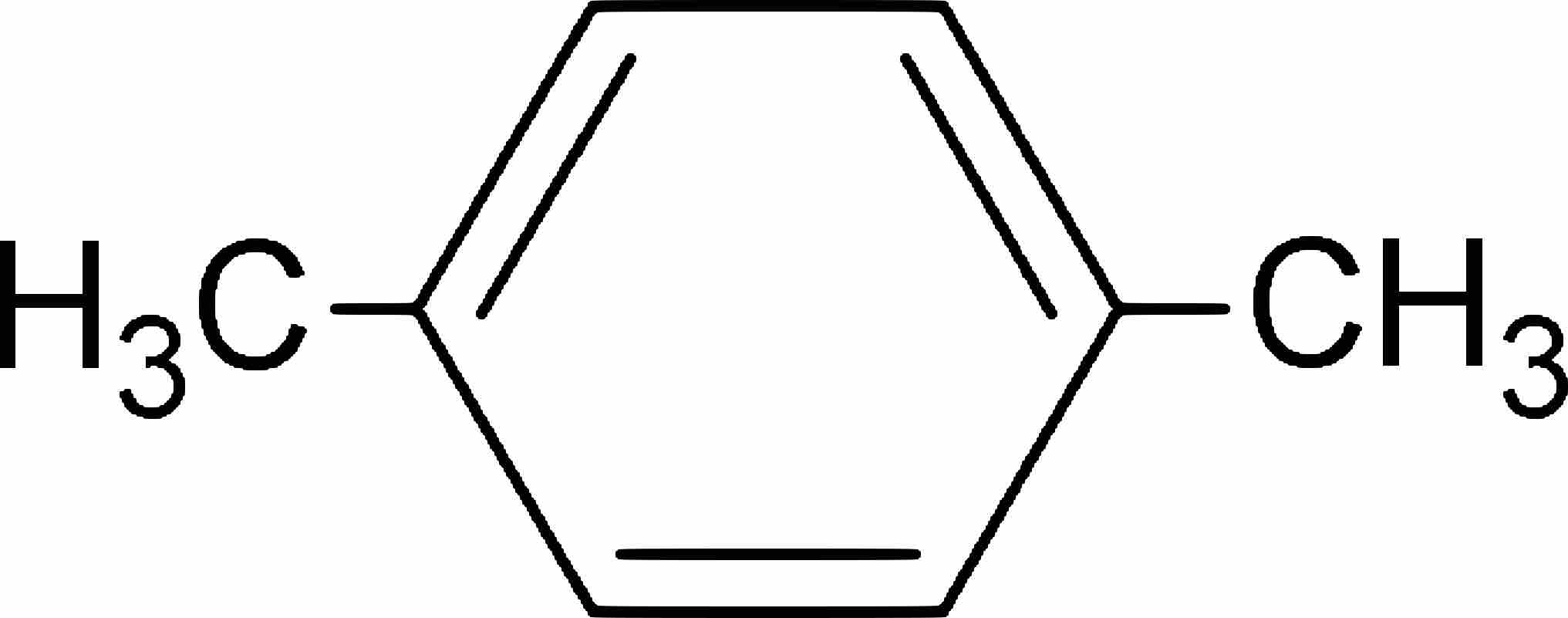
Aromatic compounds are named based on the number and type of substituents on the ring.
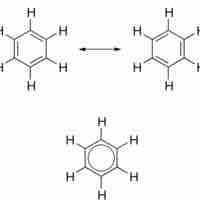
Aromatic compounds are ring structures with delocalized
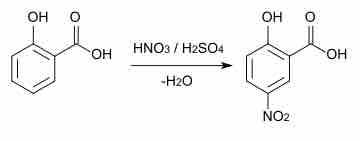
Aromatic compounds can participate in a range of reactions including substitution, coupling, and hydrogenation reactions.
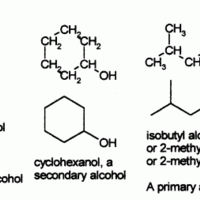
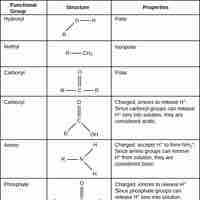
Functional groups refer to specific atoms bonded in a certain arrangement that give a compound certain physical and chemical properties.
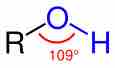
Alcohols are functional groups characterized by the presence of an -OH group.

Ethers are a class of organic compounds characterized by an oxygen atom connected to two alkyl or aryl groups.
Aldehydes and ketones are classes of organic compounds that contain a carbonyl (C=O) group.
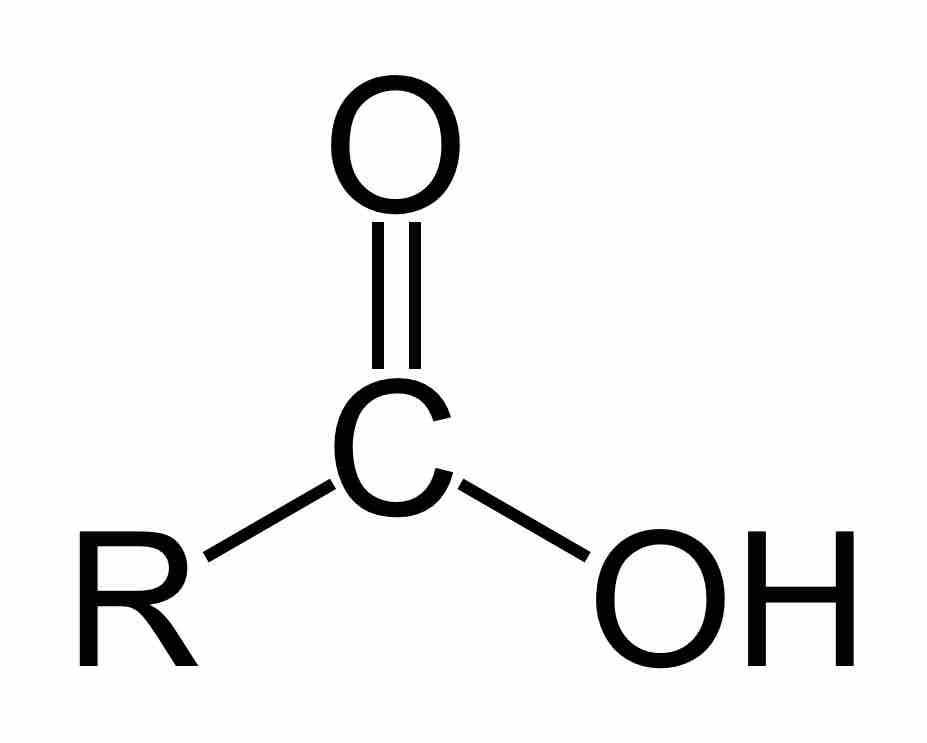
Carboxylic acids are organic acids that contain a carbon atom that participates in both a hydroxyl and a carbonyl functional group.
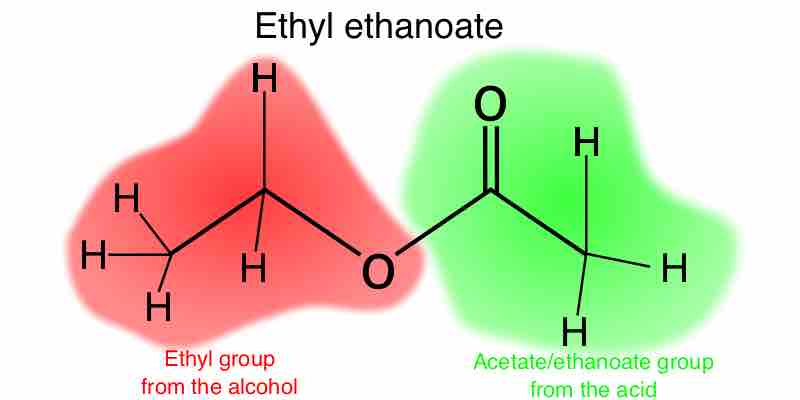
Esters are functional groups produced from the condensation of an alcohol with a carboxylic acid, and are named based on these components.
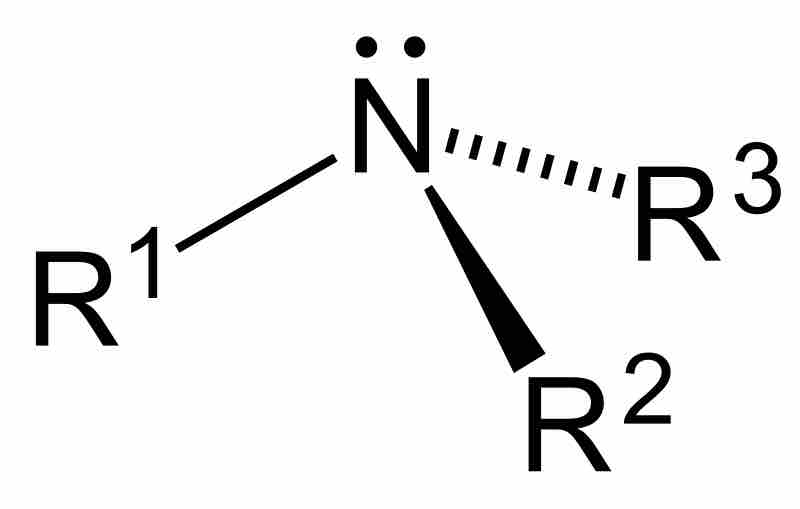
Amines are compounds characterized by the presence of a nitrogen atom, a lone pair of electrons, and three substituents.