Esters are an important functional group in organic chemistry, and they are generally written RCOOR' or RCO2R'.
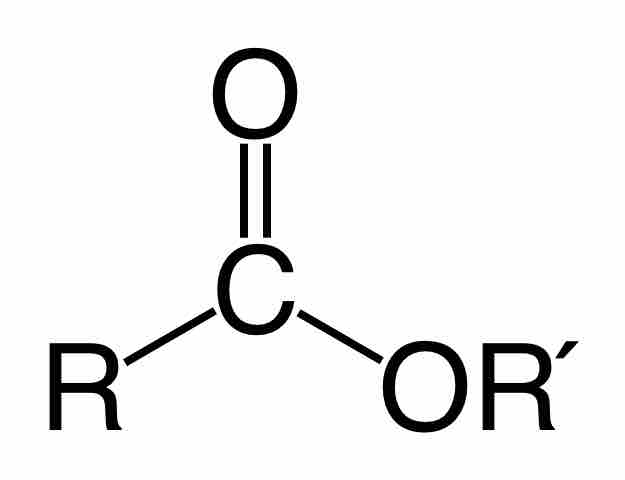
Esters
An ester is characterized by the orientation and bonding of the atoms shown, where R and R' are both carbon-initiated chains of varying length, also known as alkyl groups.
As usual, R and R' are both alkyl groups or groups initiating with carbon. Esters are derivative of carboxylic acids where the hydroxyl (OH) group has been replaced by an alkoxy (O-R) group. They are commonly synthesized from the condensation of a carboxylic acid with an alcohol:
Esters are ubiquitous. Most naturally occurring fats and oils are the fatty acid esters of glycerol. Esters are typically fragrant, and those with low enough molecular weights to be volatile are commonly used as perfumes and are found in essential oils and pheromones. Polymerized esters, or polyesters, are important plastics, with monomers linked by esteric units like this:
CO2RCO2RCO2R... etc.
Nomenclature
The word "ester" was coined in 1848 by German chemist Leopold Gmelin, probably as a contraction of the German Essigäther, meaning acetic ether.
Ester names are derived from the parent alcohol and acid. For example, the ester formed by ethanol and ethanoic acid is known as ethyl ethanoate; "ethanol" is reduced to "ethyl," while "ethanoic acid" is reduced to "ethanoate." Other examples of ester names include methyl propanoate, from methanol and propanoic acid, and butyl octanoate, from butane and octanoic acid.
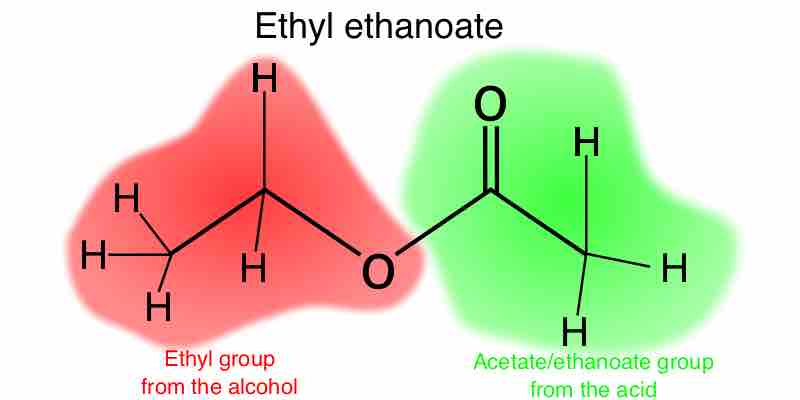
Ethyl ethanoate
The name ethyl ethanoate is derived from the components from which it is synthesized: ethanol and ethanoic acid. In this diagram, the red part of the molecule represents the portion formerly attributed to ethanol (minus a H), and the green part of the molecule represents the ethanoic acid portion (minus an OH). Esterification is a form of dehydration synthesis, so the H and OH components are removed as water.
In the case of esters formed from common carboxylic acids, more colloquial terms are sometimes used. For example, ethanoic acid is more commonly known as acetic acid, and thus its esters contain "acetate" instead of "ethanoate" in their names. Other such substitutions include "formate" instead of "methanoate," "propionate" instead of "propanoate," and "butyrate" instead of "butanoate."
The chemical formulas of organic esters are typically written in the format of RCO2R', where R and R' are the hydrocarbon parts of the carboxylic acid and alcohol, respectively. For example, butyl acetate, systematically known as ethanoic acid, is derived from butanol and acetic acid and would be written CH3CO2C4H9. Alternative presentations are common, including BuOAc and CH3COOC4H9. Cyclic esters are known as lactones.
Structure and Bonding
Esters contain a carbonyl center, which gives rise to 120 degree C-C-O and O-C-O bond angles due to sp2 hybridization. Unlike amides, esters are structurally flexible functional groups because rotation about the C-O-C bonds has a lower energy barrier. Their flexibility and low polarity affects their physical properties on a macroscopic scale; they tend to be less rigid, leading to a lower melting point, and more volatile, leading to a lower boiling point, than the corresponding amides. The pKa of the alpha-hydrogens, or the hydrogens attached to the carbon adjacent to the carbonyl, on esters is around 25, making them essentially non-acidic except in the presence of very strong bases.
Physical Properties and Characterization
Esters are more polar than ethers, but less so than alcohols. They participate in hydrogen bonds as hydrogen bond acceptors, but cannot act as hydrogen bond donors, unlike their parent alcohols and carboxylic acids. This ability to participate in hydrogen bonding confers some water-solubility, depending on the length of the alkyl chains attached. Since they have no hydrogens bonded to oxygens, as alcohols and carboxylic acids do, esters do not self-associate. Consequently, esters are more volatile than carboxylic acids of similar molecular weight.
Characterization and Analysis
Esters are usually identified by gas chromatography, taking advantage of their volatility. IR (infrared) spectra for esters feature an intense, sharp band in the range 1730–1750 cm−1 assigned to νC=O, or vibration of the C=O bond. This peak changes depending on the functional groups attached to the carbonyl. For example, a benzene ring or double bond in conjugation with the carbonyl will bring the wavenumber down to around 30 cm−1.
Reactivity
Esters react with nucleophiles at the carbonyl carbon. The carbonyl is weakly electrophilic, but is attacked by strong nucleophiles such as amines, alkoxides, hydride sources, and organolithium compounds. The carbonyl's electrophilicity can increase if it is protonated; in acidic media, an ester can be hydrolyzed by water to form a carboxylic acid and an alcohol.
The C-H bonds adjacent to the carbonyl are weakly acidic, but undergo deprotonation with strong bases. This process is the one that usually initiates condensation reactions. The carbonyl oxygen is weakly basic (less so than in amides), but can form adducts with Lewis acids.