Aromatic compounds are ring structures with unusual stability due to delocalized pi electron density that is shared between all of the carbon atoms in the ring.
There are a number of historically common names for aromatic structures. These names are frequently used in favor of standardized IUPAC nomenclature. For example, methylbenzene is often referred to as toluene, and dimethylbenzene is often called xylene.
Aromatic Compounds with a Single Substituent
When there is a single substituent on a benzene ring and the substituent contains six or fewer carbons, the substituent is included as a prefix to benzene. Alkyl groups are named according to the alkane series convention ending with -yl: methyl (for a single carbon), ethyl (for two carbons), propyl (for three carbons), etc.
If the substituent contains more than six carbons, the alkane portion is named first, and the aromatic ring portion is added as a suffix. For instance, an aromatic ring bonded to an 8-carbon chain would be 1-phenyloctane, and not octylbenzene.
Aromatic Compounds with Multiple Substituents
When there are multiple substituents, ring atoms are numbered to minimize the numbers assigned to the substituted positions.
Disubstituted benzene rings can be named based on the relative positions of the substituents: the prefix ortho- is used if the substituents occupy adjacent positions on the ring (1,2), meta- is used if the substituents are separated by one ring position (1,3), and para- if they are found on opposite sides of the ring (1,4).
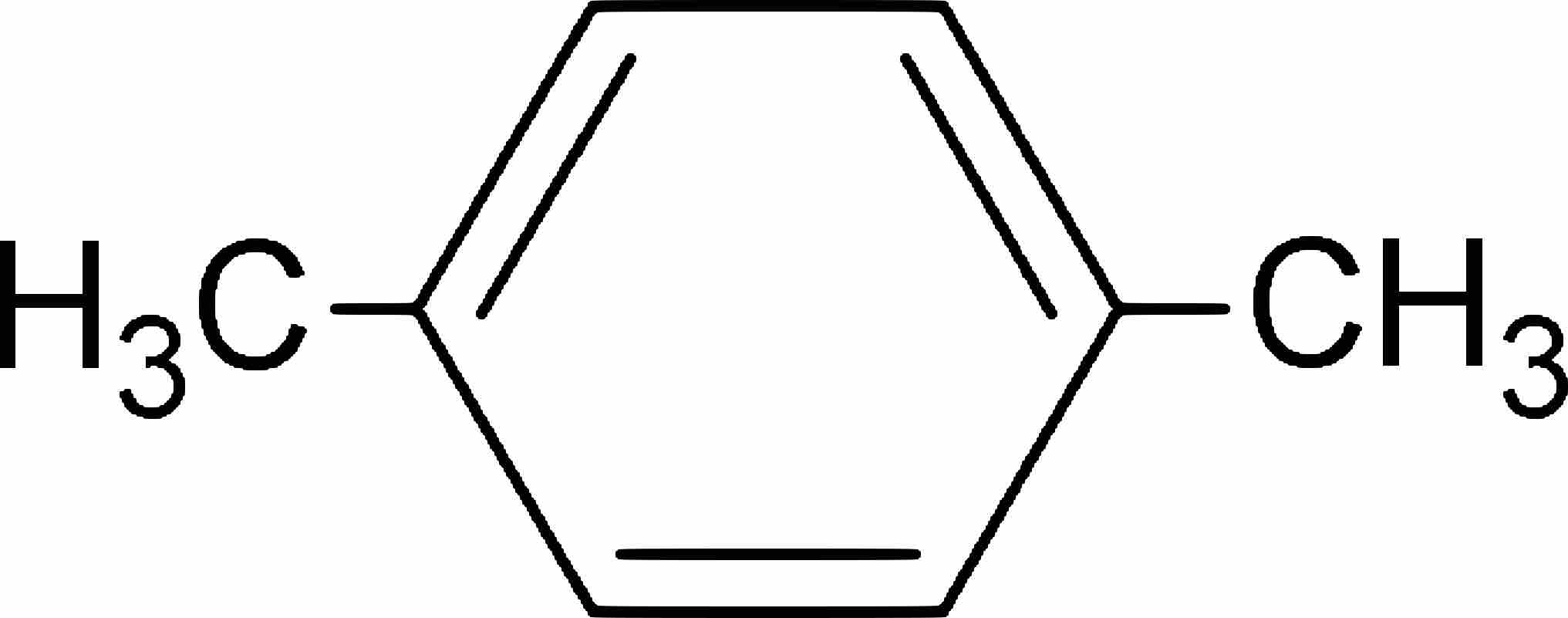
p-Xylene
Chemical structure of 1,4-dimethylbenzene, better known as para-xylene.