General Properties of Cyclic Organic Compounds
Cycloalkanes are saturated hydrocarbons that contain a ring in their carbon backbones. Analogous ring structures containing double and triple bonds are known as cycloalkenes and cycloalkynes. Cycloalkanes with one ring have the chemical formula CnH2n.
Cycloalkanes, like alkanes, are subject to intermolecular forces called London dispersion forces. These weak intermolecular interactions result in relatively low melting and boiling points. Like alkanes, cycloalkanes are not particularly reactive, with the exception of the smallest, most strained cycloalkanes.
Cycloalkane Structures
Cycloalkanes are composed of sp3 hybridized carbon and hydrogen atoms connected by sigma bonds. However, unlike linear hydrocarbons, which can achieve a more stable tetrahedral configuration around each carbon atom in the backbone, the bond angles in cycloalkanes are constrained, producing ring strain.
In order to achieve a more optimal configuration, the rings form puckered rather than flat structures. Cyclohexane forms a configuration called the chair configuration, along with a less stable boat configuration.
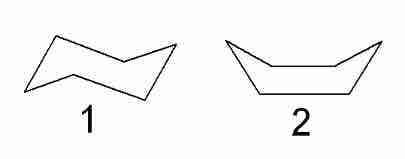
Chair and boat configurations of cyclohexane
The first image represents the chair configuration of cyclohexane, while the second represents the boat configuration.
Cycloalkane Naming or Nomenclature
Cycloalkanes are named using the same conventions as linear alkanes, with the prefix cyclo- added to the front of the name. The series includes cyclopropane, cyclobutane, cyclopentane, cyclohexane, and cycloheptane.
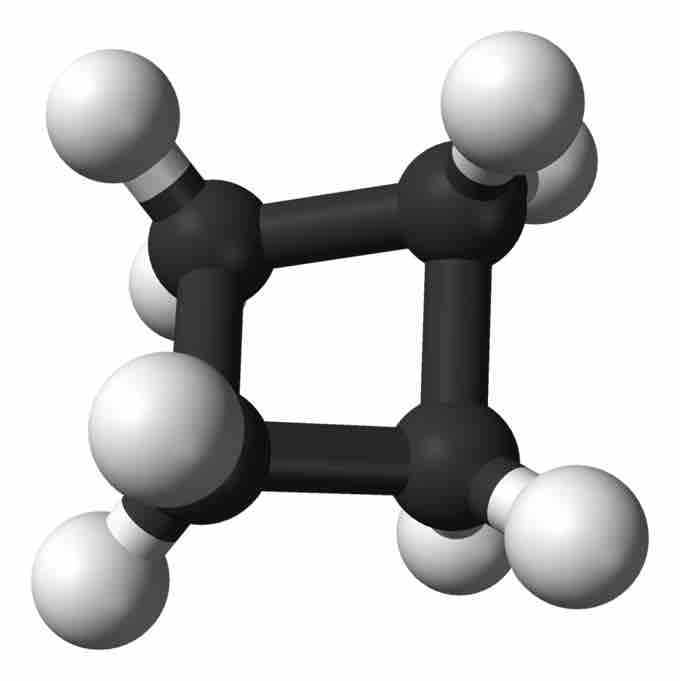
Cyclobutane
Ball-and-stick model of cyclobutane.
When naming substituted cycloalkanes, the ring positions are numbered to minimize the numbering of the appended groups. Numbering is only necessary when there is more than one substituent.
Cycloalkane Isomers
The rings of cycloalkanes have two faces, and substituent groups can be appended to either face. When two substituents occupy the same face, the configuration is known as the cis isomer, while the isomer with substituents on opposite faces is referred to as the trans isomer.
Polycyclic Compounds
Cyclic organic compounds can contain more than one ring. Hydrocarbons with two rings are called bicyclic, and well-known examples are norbornane and decalin.
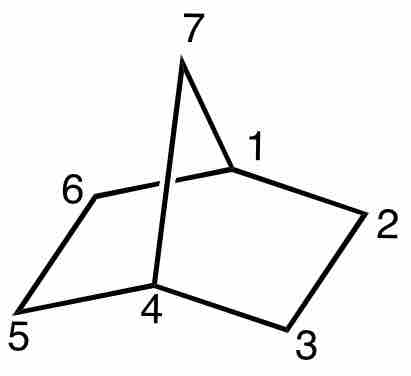
Norbornane
Notice that norbornane contains two 5-membered rings which are fused. Carbon atoms 1, 4, 7 are common to both cyclopentane rings. (Formally named: bicyclo[2.2.1]heptane).
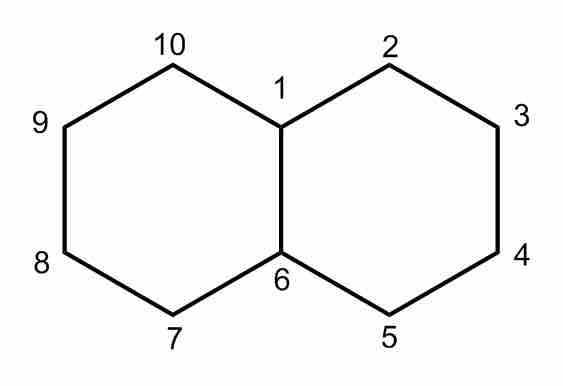
Decalin
Decalin, also known as bicyclo[4.4.0]decane. Notice that the two fused cyclohexane rings have carbons 1, 6 in common.