The Role of Functional Groups
In organic chemistry, a functional group is a specific group of atoms or bonds within a compound that is responsible for the characteristic chemical reactions of that compound. The same functional group will behave in a similar fashion, by undergoing similar reactions, regardless of the compound of which it is a part. Functional groups also play an important part in organic compound nomenclature; combining the names of the functional groups with the names of the parent alkanes provides a way to distinguish compounds.
The atoms of a functional group are linked together and to the rest of the compound by covalent bonds. The first carbon atom that attaches to the functional group is referred to as the alpha carbon; the second, the beta carbon; the third, the gamma carbon, etc. Similarly, a functional group can be referred to as primary, secondary, or tertiary, depending on if it is attached to one, two, or three carbon atoms .
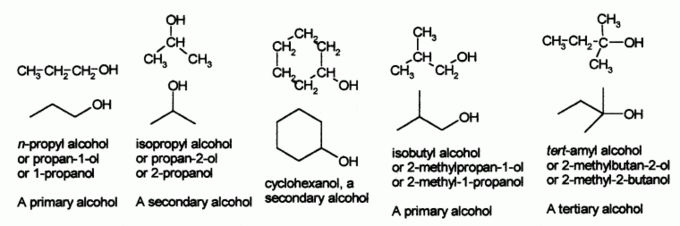
Classification of alcohols
Alcohols are a common functional group (-OH). They can be classified as primary, secondary, or tertiary, depending on how many carbon atoms the central carbon is attached to.
Functional Groups and Reactivity
Functional groups play a significant role in directing and controlling organic reactions. Alkyl chains are often nonreactive, and the direction of site-specific reactions is difficult; unsaturated alkyl chains with the presence of functional groups allow for higher reactivity and specificity. Often, compounds are functionalized with specific groups for a specific chemical reaction. Functionalization refers to the addition of functional groups to a compound by chemical synthesis. Through routine synthesis methods, any kind of organic compound can be attached to the surface. In materials science, functionalization is employed to achieve desired surface properties; functional groups can also be used to covalently link functional molecules to the surfaces of chemical devices.
In organic chemistry, the most common functional groups are carbonyls (C=O), alcohols (-OH), carboxylic acids (CO2H), esters (CO2R), and amines (NH2). It is important to be able to recognize the functional groups and the physical and chemical properties that they afford compounds.