Structure of Ethers
Ethers are a class of organic compounds that contain an ether group. An ether group is an oxygen atom connected to two alkyl or aryl groups. They follow the general formula R-O-R'. The C-O-C linkage is characterized by bond angles of 104.5 degrees, with the C-O distances being about 140 pm. The oxygen of the ether is more electronegative than the carbons. Thus, the alpha hydrogens are more acidic than in regular hydrocarbon chains.
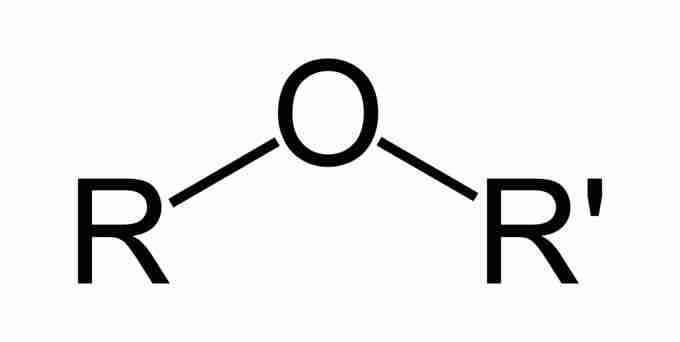
Ethers
The general structure of an ether. An ether is characterized by an oxygen bonded to two alkyl or aryl groups, represented here by R and R'. The substituents can be, but do not need to be, the same.
Nomenclature of Ethers
There are two ways to name ethers. The most common way is to identify the alkyl groups on either side of the oxygen atom in alphabetical order, then write "ether." For example, ethyl methyl ether is the ether that has an ethyl group and a methyl group on either side of the oxygen atom. If the two alkyl groups are identical, the ether is called di[alkyl] ether. For example, diethyl ether is the ether with an ethyl group on each side of the oxygen atom.
The other way of naming ethers is the formal, IUPAC method. This way, the form is: [short alkyl chain][oxy][long alkyl chain]. For example, the IUPAC name for ethyl methyl ether would be methoxyethane.
In cyclic ethers, the stem of the compound is known as a oxacycloalkane. The "oxa" is an indicator of the replacement of the carbon by an oxygen in the ring. An example is oxacyclopentane, a five-membered ring in which there are four carbon atoms and one oxygen atom.

Tetrahydrofuran (THF)
The common name of the cyclic ether "oxacyclopentane" is tetrahydrofuran, or THF. It is a common organic solvent that is miscible with water.
Properties of Ethers
Ethers are rather nonpolar due to the presence of an alkyl group on either side of the central oxygen. The presence of the bulky alkyl groups that are adjacent to it means that the oxygen atom is largely unable to participate in hydrogen bonding. Ethers, therefore, have lower boiling points compared to alcohols of similar molecular weight. However, as the alkyl chain of the ethers becomes longer, the difference in boiling points becomes smaller. This is due to the effect of increased Van der Waals interactions as the number of carbons increases, and therefore the number of electrons increases as well. The two lone pairs of electrons present on the oxygen atoms make it possible for ethers to form hydrogen bonds with water. Ethers are more polar than alkenes, but not as polar as esters, alcohols or amides of comparable structures.
Reactions
Ethers have relatively low chemical reactivity, but they are still more reactive than alkanes. Although they resist undergoing hydrolysis, they are often cleaved by acids, which results in the formation of an alkyl halide and an alcohol. Ethers tend to form peroxides in the presence of oxygen or air. The general formula is R-O-O-R'. Ethers can serve as Lewis and Bronsted bases, serving to donate electrons in reactions, or accept protons. Ethers can be formed in the laboratory through the dehydration of alcohols (2R-OH → R-O-R + H2O at high temperature), nucleophilic displacement of alkyl halides by alkoxides (R-ONa + R'-X → R-O-R' + NaX), or electrophilic addition of alcohols to alkenes (R2C=CR2 + R-OH → R2CH-C(-O-R)-R2).