The amine functional group contains a basic nitrogen atom with a lone pair of electrons. As such, the group is derivative of ammonia, in which one or more hydrogen atoms have been replaced by a carbon-containing substituent. Compounds with the nitrogen group attached to a carbonyl within the structure are referred to as amides, and they have the structure R-CO-NR'R". Amine groups bonded to an aromatic (conjugated cyclic) structure are known as aromatic amines. The aromatic structure effectively decreases the alkalinity of the amine, while the presence of the amine group significantly decreases the reactivity of the ring due to an electron donating effect. The prefix "amino-" or the suffix "-amine" is used when naming an amine compound. An organic compound with multiple amino groups is called a diamine, triamine, tetramine, etc.
Amine Structure
Amines are generally organized into categories based on their bonding environments. Amines that have one of their three hydrogen atoms replaced by an alkyl or aromatic substituent are referred to as primary amines. Secondary amines are those that have two substituents and one hydrogen bonded to a nitrogen. Tertiary amines are amines whose hydrogens have been completely replaced by organic substituents. Finally, cyclic amines are those in which the nitrogen has been incorporated into a ring structure, effectively making it either a secondary or tertiary amine. The general structure of an amine contains a nitrogen atom, a lone pair of electrons, and three substituents. However, it is possible to have four organic substituents on the nitrogen, making it an ammonium cation with a charged nitrogen center.
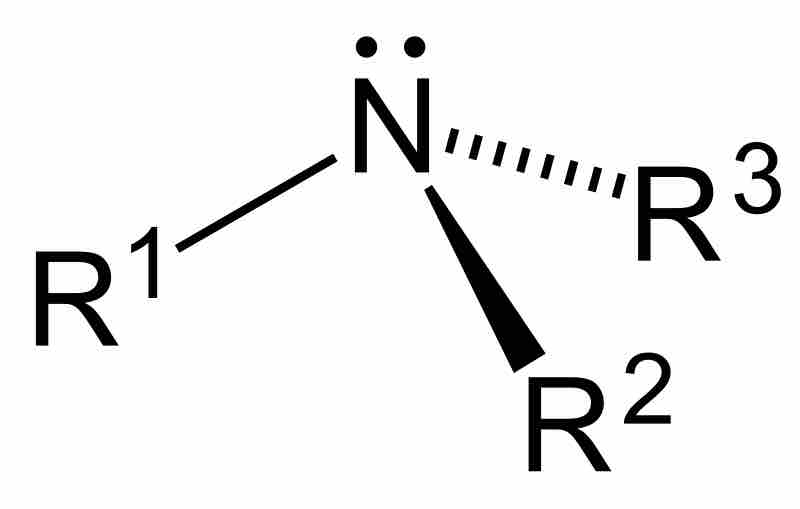
Tertiary amine
The central carbon is attached to an amine group and three other carbon atoms.
Physical Properties of Amines
Amines are able to hydrogen bond. As a result, the boiling points of these compounds are higher than those of the corresponding phosphines, but lower than those of the corresponding alcohols, which hydrogen bond to a stronger extent. Amines also display some solubility in water. However, the solubility decreases with an increase in carbon atoms, due to the increased hydrophobicity of the compound as the chain length increases. Aliphatic amines, which are amines connected to an alkyl chain, display solubility in organic polar solvents. Aromatic amines, which are amines that participate in a conjugated ring, donate their lone pair of electrons into the benzene ring, and thus their ability to engage in hydrogen bonding decreases. This results in a decrease in their solubility in water and high boiling points.
Acidity and Alkalinity of Amines
Amines of the type NHRR' and NR'R''R''' are chiral molecules and can undergo inversion. Since the barrier for inversion is quite low (~7 kcal/mol), these compounds cannot be resolved optically. Amines are bases, and their basicity depends on the electronic properties of the substituents (alkyl groups enhance the basicity; aryl groups diminish it), steric hindrance, and the degree of solvation of the protonated amine. In general, the effect of alkyl groups raises the energy of the lone pair of electrons, thus elevating the basicity. Thus, the basicity of an amine can be expected to increase with the number of alkyl groups on the amine. Additionally, the effect of the aromatic ring delocalizes the lone pair of electrons on nitrogen into the ring, resulting in decreased basicity. The solvation of protonated amines changes upon their conversion to ammonium compounds. Typically, salts of ammonium compounds exhibit the following order of solubility in water: primary ammonium (RNH3+) > secondary ammonium (R2NH2+) > tertiary ammonium (R3NH+). Quaternary ammonium salts usually exhibit the lowest solubility of the series.
Amine Preparation and Reactivity
Industrially, amines are prepared from ammonia by alkylation with alcohols. They can also be prepared via reduction of nitriles to amines using hydrogen in the presence of a nickel catalyst. Amines are quite reactive due to their basicity as well as their nucleophilicity. Most primary amines are good ligands and react with metal ions to yield coordination complexes. One of the most important reactions for amines is their formation of imines, or organic compounds where nitrogen participates in a double bond, upon reacting with ketones or aldehydes.
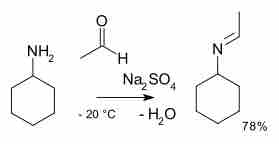
Imine formation
A primary amine is reacted with an aldehyde to produce an imine.
Applications of Amines
Amines are ubiquitous in biology. Many important molecules are amine-based, such as neurotransmitters and amino acids. Their applications in the world include being starting material for dyes and models for drug design. They are also used for gas treatment, such as removing CO2 from combustion gases.