In organic chemistry, a carbonyl group is a functional group which has a carbon double bonded to an oxygen atom: C=O.

Keto-enol tautomers
There exists an equilibrium between the ketone and the enol forms, which involves a shifting of the double bond and the movement of a proton.
Ketones
When a carbonyl functional group is placed within a molecule, it is known as a ketone. Ketones are organic compounds with the structure RC(=O)R', where R and R' can be a variety of carbon-containing substituents. IUPAC nomenclature rules dictate that ketone molecules are named by changing the suffix of the parent carbon molecule to "-one." If the position of the ketone must be specified, then a number is placed between the parent chain name and the "-one" prefix (e.g., propan-2-one), or at the beginning of the IUPAC name. The prefixes "oxo-" and "keto-" are used to describe the ketone functional group.
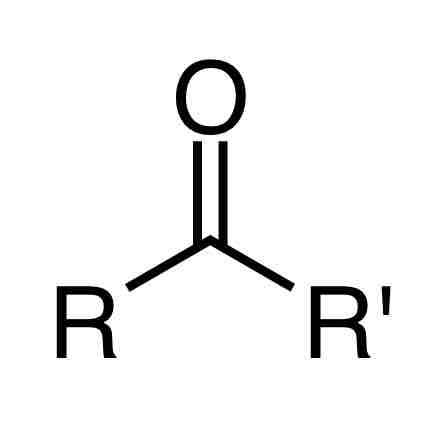
Ketone
A ketone is a type of organic compound where a carbonyl group bonds to two other carbon atoms of the carbon backbone.
The ketone carbon is sp2 hybridized, and it adopts a trigonal planar geometry around the ketonic carbon. As such, the C–C–O and C–C–C bond angles are approximately 120 degrees. Due to the carbonyl group, ketones are polar and are able to interact with other compounds through hydrogen bonding; this hydrogen bond capability makes ketones more soluble in water than related methylene compounds. Ketones are not usually hydrogen bond donors, and they tend not to exhibit intermolecular attractions with other ketones. As a result, ketones are often more volatile than alcohols and carboxylic acids of comparable molecular weights. Ketones have alpha-hydrogens which participate in keto-enol tautomerism. In the presence of a strong base, enolate formation and subsequent deprotonation of the enolate will occur.
Aldehydes
An aldehyde is an organic compound that contains a carbonyl group with the central carbon bonded to a hydrogen and R group (R-CHO). Aldehydes differ from ketones in that the carbonyl is placed at the end of the carbon skeleton rather than between two carbon atoms of the backbone. Like ketones, aldehydes are sp2 hybridized and can exist in the keto or enol tautomer. Aldehydes are named by dropping the suffix of the parent molecule, and adding the suffix "-al." For instance, a three-carbon chain with an aldehyde group on a terminal carbon would be propanal. If there are higher order functional groups on the compound, the prefix "oxo-" can be used to indicate which carbon atom is part of the aldehyde group. If the location of the aldehyde must be specified, a number can be used in between the parent chain and suffix, or at the beginning of the compound name.
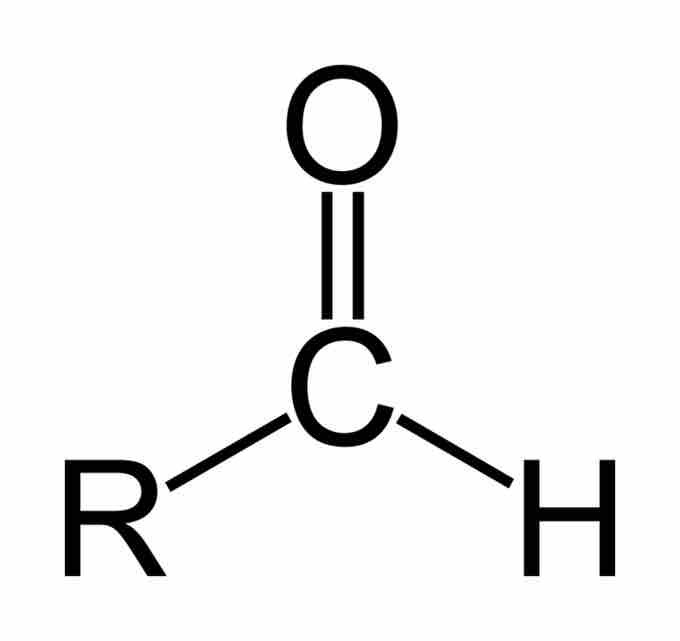
Aldehyde
An aldehyde is characterized by the presence of a carbonyl functional group at the end of a compound's carbon skeleton.
Similarities of Aldehydes and Ketones
Both aldehydes and ketones exist in an equilibrium with their enol forms; the enol form is defined as an alkene with a hydroxyl group affixed to one of the carbon atoms composing the double bond. The keto form predominates at equilibrium for most ketones. However, the enol form is important for some reactions because the deprotonated enolate form is a strong nucleophile. The equilibrium is strongly thermodynamically driven, and at room temperature the keto form is favored. The interconversion can be catalyzed by the presence of either an acid or a base.
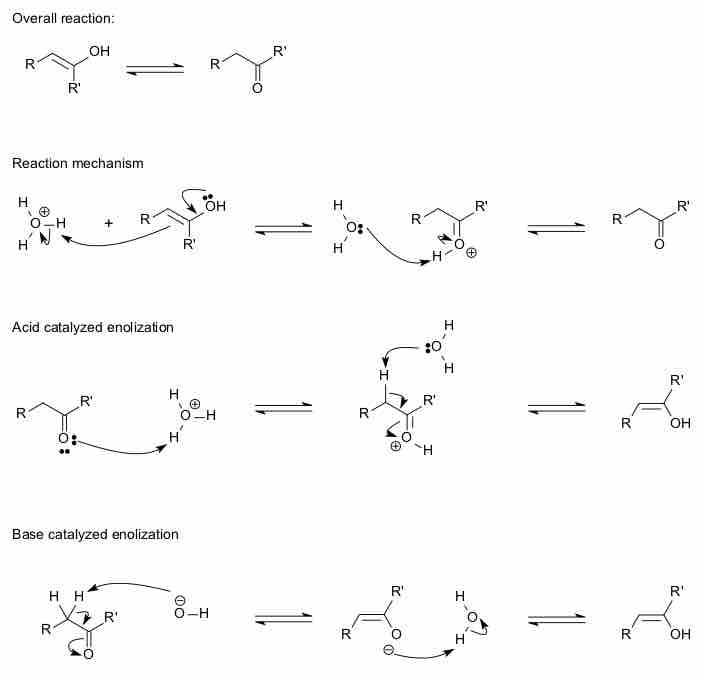
Keto-enol tautomerism
The interconversion between the two forms can be catalyzed by an acid or a base.
Ketone and Aldehyde Spectroscopy
Both ketones and aldehydes can be identified by spectroscopic methods. They display strong CO absorption bands near 1700 cm-1. In NMR spectroscopy, the carbonyl hydrogen shows a strong absorption peak, and any coupling to protons on the alpha carbon will also show strong signals.
Ketones and aldehydes can both be readily reduced to alcohols, usually in the presence of a strong reducing agent such as sodium borohydride. In the presence of strong oxidizing agents, they can be oxidized to carboxylic acids. As electrophiles, they are subject to attack by nucleophiles, meaning they participate in many nucleophilic addition reactions.