Alcohols are organic compounds in which the hydroxyl functional group (-OH) is bound to a carbon atom. Alcohols are an important class of molecules with many scientific, medical, and industrial uses.
Nomenclature of Alcohols
According to the IUPAC nomenclature system, an alcohol is named by dropping the terminal "-e" of the parent carbon chain (alkane, alkene, or alkyne in most cases) and the addition of "-ol" as the ending. If the location of the hydroxyl group must be specified, a number is inserted between the parent alkane name and the "-ol" (propan-1-ol) or before the IUPAC name (1-propanol). If a higher priority group is present, such as an aldehyde, ketone or carboxylic acid, then it is necessary to use the prefix "hydroxy-" instead of the ending "-ol."
Alcohols are classified as primary, secondary, or tertiary, based upon the number of carbon atoms connected to the carbon atom that bears the hydroxyl group.
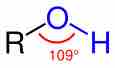
The alcohol functional group
Alcohols are characterized by the presence of an -OH group, which is generally in a bent shape, like that of water.
Structure and Physical Properties of Alcohols
The structure of an alcohol is similar to that of water, as it has a bent shape. This geometrical arrangement reflects the effect of electron repulsion and the increasing steric bulk of the substituents on the central oxygen atom. Like water, alcohols are polar, containing an unsymmetrical distribution of charge between the oxygen and hydrogen atoms. The high electronegativity of the oxygen compared to carbon leads to the shortening and strengthening of the -OH bond. The presence of the -OH groups allows for hydrogen bonding with other -OH groups, hydrogen atoms, and other molecules. Since alcohols are able to hydrogen bond, their boiling points are higher than those of their parent molecules.
Alcohols are able to participate in many chemical reactions. They often undergo deprotonation in the presence of a strong base. This weak acid behavior results in the formation in an alkoxide salt and a water molecule. Hydroxyl groups alone are not considered good leaving groups. Often, their participation in nucleophilic substitution reactions is instigated by the protonation of the oxygen atom, leading to the formation a water moiety—a better leaving group. Alcohols can react with carboxylic acids to form an ester, and they can be oxidized to aldehydes or carboxylic acids.
Alcohols have many uses in our everyday world. They are found in beverages, antifreeze, antiseptics, and fuels. They can be used as preservatives for specimens in science, and they can be used in industry as reagents and solvents because they display an ability to dissolve both polar and non-polar substances.