Thorium
About this schools Wikipedia selection
SOS Children offer a complete download of this selection for schools for use on schools intranets. Click here to find out about child sponsorship.
| Thorium | |||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
90Th
|
|||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||
| Appearance | |||||||||||||||||||||||||||||||||||||||||||
silvery, often with black tarnish |
|||||||||||||||||||||||||||||||||||||||||||
| General properties | |||||||||||||||||||||||||||||||||||||||||||
| Name, symbol, number | thorium, Th, 90 | ||||||||||||||||||||||||||||||||||||||||||
| Pronunciation | / ˈ θ ɔər i ə m / THOHR-ee-əm |
||||||||||||||||||||||||||||||||||||||||||
| Element category | actinide | ||||||||||||||||||||||||||||||||||||||||||
| Group, period, block | n/a, 7, f | ||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight | 232.03806 | ||||||||||||||||||||||||||||||||||||||||||
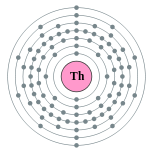
| Electron configuration | [Rn] 6d2 7s2 2, 8, 18, 32, 18, 10, 2 |
||||||||||||||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||||||||||||||
| Discovery | Jöns Jakob Berzelius (1829) | ||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||
| Phase | solid | ||||||||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | 11.7 g·cm−3 | ||||||||||||||||||||||||||||||||||||||||||
| Melting point | 2115 K, 1842 °C, 3348 °F | ||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 5061 K, 4788 °C, 8650 °F | ||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 13.81 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 514 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||
| Molar heat capacity | 26.230 J·mol−1·K−1 | ||||||||||||||||||||||||||||||||||||||||||
| Vapor pressure | |||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | 4, 3, 2, 1 (weakly basic oxide) |
||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | 1.3 (Pauling scale) | ||||||||||||||||||||||||||||||||||||||||||
| Ionization energies | 1st: 587 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||
| 2nd: 1110 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||||||||
| 3rd: 1930 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | 179 pm | ||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 206±6 pm | ||||||||||||||||||||||||||||||||||||||||||
| Miscellanea | |||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | face-centered cubic | ||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | paramagnetic | ||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | (0 °C) 147 nΩ·m | ||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 54.0 W·m−1·K−1 | ||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | (25 °C) 11.0 µm·m−1·K−1 | ||||||||||||||||||||||||||||||||||||||||||
| Speed of sound (thin rod) | (20 °C) 2490 m·s−1 | ||||||||||||||||||||||||||||||||||||||||||
| Young's modulus | 79 GPa | ||||||||||||||||||||||||||||||||||||||||||
| Shear modulus | 31 GPa | ||||||||||||||||||||||||||||||||||||||||||
| Bulk modulus | 54 GPa | ||||||||||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.27 | ||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 3.0 | ||||||||||||||||||||||||||||||||||||||||||
| Vickers hardness | 350 MPa | ||||||||||||||||||||||||||||||||||||||||||
| Brinell hardness | 400 MPa | ||||||||||||||||||||||||||||||||||||||||||
| CAS registry number | 7440-29-1 | ||||||||||||||||||||||||||||||||||||||||||
| Most stable isotopes | |||||||||||||||||||||||||||||||||||||||||||
| Main article: Isotopes of thorium | |||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||
Thorium is a naturally occurring radioactive chemical element with the symbol Th and atomic number 90. It was discovered in 1828 by the Norwegian mineralogist Morten Thrane Esmark and identified by the Swedish chemist Jöns Jakob Berzelius and named after Thor, the Norse god of thunder.
Thorium produces a radioactive gas, radon-220, as one of its decay products. Secondary decay products of thorium include radium and actinium. In nature, virtually all thorium is found as thorium-232, which undergoes alpha decay with a half-life of about 14.05 billion years. Other isotopes of thorium are short-lived intermediates in the decay chains of higher elements, and only found in trace amounts. Thorium is estimated to be about three to four times more abundant than uranium in the Earth's crust, and is chiefly refined from monazite sands as a by-product of extracting rare earth metals.
Thorium was once commonly used as the light source in gas mantles and as an alloying material, but these applications have declined due to concerns about its radioactivity. Thorium is also used as an alloying element in nonconsumable TIG welding electrodes.
Canada, China, Germany, India, the Netherlands, the United Kingdom, and the United States have experimented with using thorium as a substitute nuclear fuel in nuclear reactors. When compared to uranium, there is a growing interest in developing a thorium fuel cycle due to its greater safety benefits, absence of non- fertile isotopes, and its higher occurrence and availability. India's three stage nuclear power programme is possibly the most well known and well funded of such efforts.
Characteristics
Physical properties
Pure thorium is a silvery-white metal which is air-stable and retains its luster for several months. When contaminated with the oxide, thorium slowly tarnishes in air, becoming gray and finally black. The physical properties of thorium are greatly influenced by the degree of contamination with the oxide.
The purest specimens often contain several tenths of a percent of the oxide. Pure thorium is soft, very ductile, and can be cold-rolled, swaged, and drawn. Thorium is dimorphic, changing at 1360 °C from a face-centered cubic to a body-centered cubic structure; a body-centered tetragonal lattice form exists at high pressure with impurities driving the exact transition temperatures and pressures.
Powdered thorium metal is often pyrophoric and requires careful handling. When heated in air, thorium metal turnings ignite and burn brilliantly with a white light. Thorium has one of the largest liquid temperature ranges of any element, with 2946 °C between the melting point and boiling point. Thorium metal is paramagnetic with a ground state of 6d27s2.
Chemical properties
Thorium is slowly attacked by water, but does not dissolve readily in most common acids, except hydrochloric acid. It dissolves in concentrated nitric acid containing a small amount of catalytic fluoride ion.
Thorium's oxide is ThO2. Thorium's most comon oxidation state is +4, as in ThF4, but thorium also has an oxidation state of +3, as in ThI3. Thorium has been shown to activate carbon-hydrogen bonds, forming unusual compounds. Thorium atoms can also bond to more atoms than any other element. For instance, in the compound thorium tetrakisaminodiborane thorium bonds to fifteen hydrogen atoms.
Compounds
Thorium compounds are stable in the +4 oxidation state.
Thorium dioxide has the highest melting point (3300 °C) of all oxides.
Thorium(IV) nitrate and thorium(IV) fluoride are known in their hydrated forms: Th(NO3)4·4H2O and ThF4·4H2O, respectively. Thorium(IV) carbonate, Th(CO3)2, is also known.
When treated with potassium fluoride and hydrofluoric acid, Th4+ forms the complex anion ThF2−
6, which precipitates as an insoluble salt, K2ThF6.
Thorium(IV) hydroxide, Th(OH)4, is highly insoluble in water, and is not amphoteric. The peroxide of thorium, ThO4 or Th(O2)2, is rare in being an insoluble solid. This property can be used to separate thorium from other ions in solution.
In the presence of phosphate anions, Th4+ forms precipitates of various compositions, which are insoluble in water and acid solutions.
Thorium monoxide has recently been produced through laser ablation of thorium in the presence of oxygen. This highly polar molecule has the largest known internal electric field.
Isotopes
Twenty-seven radioisotopes have been characterized, with a range in atomic weight from 210 u (210Th) to 236 u (236Th). The most stable isotopes are:
- 232Th with a half-life of 14.05 billion years, it represents all but a trace of naturally occurring thorium.
- 230Th with a half-life of 75,380 years. Occurs as the daughter product of 238U decay.
- 229Th with a half-life of 7340 years. It has a nuclear isomer (or metastable state) with a remarkably low excitation energy of 7.6 eV.
- 228Th with a half-life of 1.92 years.
All of the remaining radioactive isotopes have half-lives that are less than thirty days and the majority of these have half-lives that are less than ten minutes.
Applications
Thorium
Thorium is a component of the magnesium alloy series, called Mag-Thor, used in aircraft engines and rockets and imparting high strength and creep resistance at elevated temperatures. Thoriated magnesium was used to build the CIM-10 Bomarc missile, although concerns about radioactivity have resulted in several missiles being removed from public display.
Thorium is also used in its oxide form (thoria) in gas tungsten arc welding (GTAW) to increase the high-temperature strength of tungsten electrodes and improve arc stability. The electrodes labeled EWTH-1 contain 1% thoria, while the EWTH-2 contain 2%. In electronic equipment, thorium coating of tungsten wire improves the electron emission of heated cathodes.
Thorium is a very effective radiation shield, although it has not been used for this purpose as much as lead or depleted uranium. Uranium-thorium age dating has been used to date hominid fossils, seabeds, and mountain ranges.
Environmental concerns related to radioactivity led to a sharp decrease in demand for nonnuclear uses of thorium in the 2000s.
Thorium compounds
Thorium dioxide (ThO2) and thorium nitrate (Th(NO3)4) were used in mantles of portable gas lights, including natural gas lamps, oil lamps and camping lights. These mantles glow with an intense white light (unrelated to radioactivity) when heated in a gas flame, and its colour could be shifted to yellow by addition of cerium.
Thorium dioxide is a material for heat-resistant ceramics, e.g., for high-temperature laboratory crucibles. When added to glass, it helps increase refractive index and decrease dispersion. Such glass finds application in high-quality lenses for cameras and scientific instruments. The radiation from these lenses can self-darken (yellow) them over a period of years and degrade film, but the health risks are minimal. Yellowed lenses may be restored to their original colorless state with lengthy exposure to intense UV light.
Thorium dioxide was used to control the grain size of tungsten metal used for spirals of electric lamps. Thoriated tungsten elements are found in the filaments of magnetron tubes. Thorium is added because of its ability to emit electrons at relatively low temperatures when heated in vacuum. Those tubes generate microwave frequencies and are applied in microwave ovens and radars.
Thorium dioxide has been used as a catalyst in the conversion of ammonia to nitric acid, in petroleum cracking and in producing sulfuric acid. It is the active ingredient of Thorotrast, which was used as radiocontrast agent for X-ray diagnostics. This use has been abandoned due to its carcinogenic nature.
Despite its radioactivity, thorium fluoride (ThF4) is used as an antireflection material in multilayered optical coatings. It has excellent optical transparency in the range 0.35–12 µm, and its radiation is primarily due to alpha particles, which can be easily stopped by a thin cover layer of another material. Thorium fluoride was also used in manufacturing carbon arc lamps, which provided high-intensity illumination for movie projectors and search lights.
Thorium as a nuclear fuel
Benefits and challenges
The naturally occurring isotope thorium-232 is a fertile material, and with a suitable neutron source can be used as nuclear fuel in nuclear reactors, including breeder reactors. In 1997, the U.S. Energy Department underwrote research into thorium fuel, and research also was begun in 1996 by the International Atomic Energy Agency (IAEA), to study the use of thorium reactors. Nuclear scientist Alvin Radkowsky of Tel Aviv University in Israel founded a consortium to develop thorium reactors, which included other companies: Raytheon Nuclear Inc., Brookhaven National Laboratory, and the Kurchatov Institute in Moscow.
Radkowsky was chief scientist in the U.S. nuclear submarine program directed by Admiral Hyman Rickover and later headed the design team which built the USA's first civilian nuclear power plant at Shippingport, Pennsylvania, which was a scaled-up version of the first naval reactor. The third Shippingport core, initiated in 1977, bred thorium. Even earlier examples of reactors using fuel with thorium exist, including the first core at the Indian Point Energy Centre in 1962.
Some countries, including India, are now investing in research to build thorium-based nuclear reactors. A 2005 report by the International Atomic Energy Agency discusses potential benefits along with the challenges of thorium reactors. India has also made thorium-based nuclear reactors a priority with its focus on developing fast breeder technology.
Some benefits of thorium fuel when compared with uranium were summarized as follows:
- Weapons-grade fissionable material (233U) is harder to retrieve safely and clandestinely from a thorium reactor;
- Thorium produces 10 to 10,000 times less long-lived radioactive waste;
- Thorium mining produces a single pure isotope, whereas the mixture of natural uranium isotopes must be enriched to function in most common reactor designs. The same cycle could also use the fissionable U-238 component of the natural uranium, and also contained in the depleted reactor fuel;
- Thorium cannot sustain a nuclear chain reaction without priming, so fission stops by default in an accelerator driven reactor.
When used in a breeder-like reactor, however, unlike uranium-based light water reactors, thorium requires irradiation and reprocessing before the above-noted advantages of thorium-232 can be realized, which initially makes solid thorium fuels more expensive than uranium fuels. But experts note that "the second thorium reactor may activate a third thorium reactor. This could continue in a chain of reactors for a millennium if we so choose." They add that because of thorium's abundance, it will not be exhausted in 1,000 years.
The Thorium Energy Alliance (TEA), an educational advocacy organization, emphasizes that "there is enough thorium in the United States alone to power the country at its current energy level for over 10,000 years."
Thorium energy fuel cycle
Like 238U, 232Th is not fissile itself, but it is fertile: it will absorb slow neutrons to produce, after two beta decays, 233U, which is fissile. Also, preparation of thorium fuel does not require isotopic separation.
The thorium fuel cycle creates 233U, which, if separated from the reactor's fuel, could with some difficulty be used for making nuclear weapons. This is one reason why a liquid-fuel cycle (e.g., Molten Salt Reactor or MSR) is preferred — only a limited amount of 233U ever exists in the reactor and its heat-transfer systems, preventing any access to weapons material; however the neutrons produced by the reactor can be absorbed by a thorium or uranium blanket and fissile 233U or 239Pu produced. Also, the 233U could be continuously extracted from the molten fuel as the reactor is running. Neutrons from the decay of uranium-233 can be fed back into the fuel cycle to start the cycle again.
The neutron flux from spontaneous fission of 233U is negligible. 233U can thus be used easily in a simple gun-type nuclear bomb design. In 1977, a light-water reactor at the Shippingport Atomic Power Station was used to establish a 232Th-233U fuel cycle. The reactor worked until its decommissioning in 1982. Thorium can be and has been used to power nuclear energy plants using both the modified traditional Generation III reactor design and prototype Generation IV reactor designs. The use of thorium as an alternative fuel is one innovation being explored by the International Project on Innovative Nuclear Reactors and Fuel Cycles (INPRO), conducted by the International Atomic Energy Agency (IAEA).
Unlike its use in Molten salt reactors, when using solid thorium in modified light water reactor (LWR) problems include: the undeveloped technology for fuel fabrication; in traditional, once-through LWR designs potential problems in recycling thorium due to highly radioactive 228Th; some weapons proliferation risk due to production of 233U; and the technical problems (not yet satisfactorily solved) in reprocessing. Much development work is still required before the thorium fuel cycle can be commercialized for use in LWR. The effort required has not seemed worth it while abundant uranium is available.
Commercial nuclear power station
India's Kakrapar-1 reactor is the world's first reactor which uses thorium rather than depleted uranium to achieve power flattening across the reactor core. India, which has about 25% of the world's thorium reserves, is developing a 300 MW prototype of a thorium-based Advanced Heavy Water Reactor (AHWR). The prototype is expected to be fully operational by 2013, after which five more reactors will be constructed. The reactor is a fast breeder reactor and uses a plutonium core rather than an accelerator to produce neutrons. As accelerator-based systems can operate at sub-criticality they could be developed too, but that would require more research. India currently envisages meeting 30% of its electricity demand through thorium-based reactors by 2050.
Existing thorium energy projects
The German THTR-300 was the first commercial power station powered almost entirely with thorium. India's 300 MWe AHWR (pressurized heavy water reactor) reactor began construction in 2011. The design envisages a start up with reactor grade plutonium which will breed U-233 from Th-232. After that the input will only be thorium for the rest of the reactor's design life.
The primary fuel of the HT3R Project near Odessa, Texas, USA will be ceramic-coated thorium beads. The earliest date the reactor will become operational is in 2015.
Best results occur with molten salt reactors (MSRs), such as ORNL's liquid fluoride thorium reactor (LFTR), which have built-in negative-feedback reaction rates due to salt expansion and thus reactor throttling via load. This is a great safety advantage, since no emergency cooling system is needed, which is both expensive and adds thermal inefficiency. In fact, an MSR was chosen as the base design for the 1960s DoD nuclear aircraft largely because of its great safety advantages, even under aircraft maneuvering. In the basic design, an MSR generates heat at higher temperatures, continuously, and without refuelling shutdowns, so it can provide hot air to a more efficient ( Brayton Cycle) turbine. An MSR run this way is about 30% better in thermal efficiency than common thermal plants, whether combustive or traditional solid-fuelled nuclear.
In 2009, United States Congressman Joe Sestak unsuccessfully attempted to secure funding for research and development of a destroyer-sized reactor using thorium-based liquid fuel.
CANDU reactors of Atomic Energy Canada Limited are capable of using thorium as a fuel source.
At the 2011 annual conference of the Chinese Academy of Sciences it was announced that "China has initiated a research and development project in thorium molten-salt reactor technology."
Projects combining uranium and thorium
Fort St. Vrain Generating Station, a demo HTGR in Colorado, USA, operating from 1977 until 1992, employed enriched uranium fuel that also contained thorium. This resulted in high fuel efficiency because the thorium was converted to uranium and then burnt.
History
Morten Thrane Esmark found a black mineral on Løvøya island, Norway and gave a sample to his father Jens Esmark, a noted mineralogist. The elder Esmark was not able to identify it and sent a sample to Swedish chemist Jöns Jakob Berzelius for examination in 1828. Berzelius determined that it contained a new element, which he named thorium after Thor, the Norse god of thunder. He published his findings in 1829. Berzelius reused the name of a previous element discovery from a mineral from the Falun which later proved to be a yttrium mineral. The metal had no practical uses until Carl Auer von Welsbach invented the gas mantle in 1885.
Thorium was first observed to be radioactive in 1898, independently, by Polish-French physicist Marie Curie and German chemist Gerhard Carl Schmidt. Between 1900 and 1903, Ernest Rutherford and Frederick Soddy showed how thorium decayed at a fixed rate over time into a series of other elements. This observation led to the identification of half-life as one of the outcomes of the alpha particle experiments that led to their disintegration theory of radioactivity.
The crystal bar process (or "iodide process") was discovered by Anton Eduard van Arkel and Jan Hendrik de Boer in 1925 to produce high-purity metallic thorium.
The name ionium was given early in the study of radioactive elements to the 230Th isotope produced in the decay chain of 238U before it was realized that ionium and thorium were chemically identical. The symbol Io was used for this supposed element.
Thorium-232 is a primordial nuclide, having existed in its current form for over 4.5 billion years, predating the formation of the Earth; it was forged in the cores of dying stars through the r-process and scattered across the galaxy by supernovas. Its radioactive decay produces a significant amount of the earth's internal heat.
Occurrence
Thorium is found in small amounts in most rocks and soils; it is three times more abundant than tin in the Earth's crust and is about as common as lead. Soil commonly contains an average of around 6 parts per million (ppm) of thorium. Thorium occurs in several minerals including thorite (ThSiO4), thorianite (ThO2 + UO2) and monazite. Thorianite is a rare mineral and may contain up to about 12% thorium oxide. Monazite contains 2.5% thorium, allanite has 0.1 to 2% thorium and zircon can have up to 0.4% thorium. Thorium-containing minerals occur on all continents. Thorium is several times more abundant in Earth's crust than all isotopes of uranium combined and thorium-232 is several hundred times more abundant than uranium-235.
232Th decays very slowly (its half-life is comparable to the age of the universe) but other thorium isotopes occur in the thorium and uranium decay chains. Most of these are short-lived and hence much more radioactive than 232Th, though on a mass basis they are negligible.
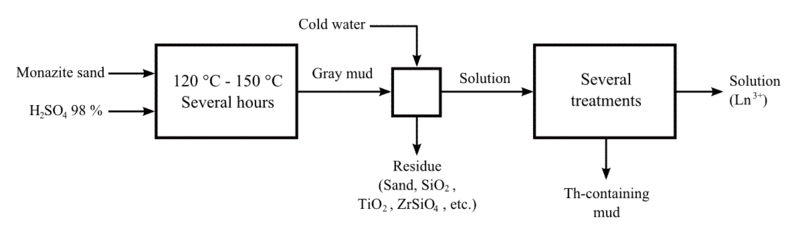
Thorium extraction
Thorium has been extracted chiefly from monazite through a complex multi-stage process. The monazite sand is dissolved in hot concentrated sulfuric acid (H2SO4). Thorium is extracted as an insoluble residue into an organic phase containing an amine. Next it is separated or stripped using an ion such as nitrate, chloride, hydroxide, or carbonate, returning the thorium to an aqueous phase. Finally, the thorium is precipitated and collected.
Several methods are available for producing thorium metal: it can be obtained by reducing thorium oxide with calcium, by electrolysis of anhydrous thorium chloride in a fused mixture of sodium and potassium chlorides, by calcium reduction of thorium tetrachloride mixed with anhydrous zinc chloride, and by reduction of thorium tetrachloride with an alkali metal.
Reserve estimates
Present knowledge of the distribution of thorium resources is poor because of the relatively low-key exploration efforts arising out of insignificant demand. There are two sets of estimates that define world thorium reserves, one set by the US Geological Survey (USGS) and the other supported by reports from the OECD and the International Atomic Energy Agency (the IAEA). Under the USGS estimate, USA, Australia, and India have particularly large reserves of thorium.
Both the IAEA and OECD appear to conclude that India may possess the lion's share of world's thorium deposits. The Government of India's latest estimate, shared in the country's Parliament in August 2011, puts the recoverable reserve at 846,477 tonnes.
India and Australia are believed to possess about 300,000 tonnes each; i.e. each country possessing 25% of the world's thorium reserves. In the OECD reports, however, estimates of Australia's Reasonably Assured Reserves (RAR) of thorium indicate only 19,000 tonnes and not 300,000 tonnes as indicated by USGS. The two sources vary wildly for countries such as Brazil, Turkey, and Australia, however, both reports appear to show some consistency with respect to India's thorium reserve figures, with 290,000 tonnes (USGS) and 319,000 tonnes (OECD/IAEA).
The IAEA's 2005 report estimates India's reasonably assured reserves of thorium at 319,000 tonnes, but mentions recent reports of India's reserves at 650,000 tonnes.
The prevailing estimate of the economically available thorium reserves comes from the U.S. Geological Survey, Mineral Commodity Summaries (1996–2010):
| Country | Reserves |
|---|---|
| India | 650,000 |
| United States | 440,000 |
| Australia | 300,000 |
| Brazil | 16,000 |
| Canada | 100,000 |
| Malaysia | 4,500 |
| South Africa | 35,000 |
| Other Countries | 90,000 |
| World Total | 1,660,000 |
Note: The OECD/NEA report notes that the estimates (that the Australian figures are based on) are subjective, due to the variability in the quality of the data, a lot of which is old and incomplete. Adding to the confusion are subjective claims made by the Australian government (in 2009, through its Geoscience department) that combine the reasonably assured reserves (RAR) estimates with "inferred" data (i.e. subjective guesses). This strange combined figure of RAR and "guessed" reserves yields a figure, published by the Australian government, of 489,000 tonnes, however, using the same criteria for Brazil or India would yield reserve figures of between 600,000 to 1,300,000 tonnes for Brazil and between 300,000 to 600,000 tonnes for India. Irrespective of isolated claims by the Australian government, the most credible third-party and multi-lateral reports, those of the OECD/IAEA and the USGS, consistently report high thorium reserves for India while not doing the same for Australia.
Another estimate of reasonably assured reserves (RAR) and estimated additional reserves (EAR) of thorium comes from OECD/NEA, Nuclear Energy, "Trends in Nuclear Fuel Cycle", Paris, France (2001):
| Country | RAR Th | EAR Th |
|---|---|---|
| India | 519,000 | 21% |
| Australia | 489,000 | 19% |
| USA | 400,000 | 13% |
| Turkey | 344,000 | 11% |
| Venezuela | 302,000 | 10% |
| Brazil | 302,000 | 10% |
| Norway | 132,000 | 4% |
| Egypt | 100,000 | 3% |
| Russia | 75,000 | 2% |
| Greenland | 54,000 | 2% |
| Canada | 44,000 | 2% |
| South Africa | 18,000 | 1% |
| "Other countries" | 33,000 | 2% |
| "World total" | 2,810,000 |
The preceding reserve figures refer to the amount of thorium in high-concentration deposits inventoried so far and estimated to be extractable at current market prices; millions of times more total exist in Earth's 3 * 1019 ton crust, around 120 trillion tons of thorium, and lesser but vast quantities of thorium exist at intermediate concentrations. Proved reserves are "a poor indicator of the total future supply of a mineral resource."
The Lemhi Pass, along the Idaho- Montana border, has one of the world's largest known high quality thorium deposits. Thorium Energy, Inc. has the mineral rights to approximately 1360 acres (5.5 sq km) of it and states that they have proven thorium oxide reserves of 600 thousand tons and probable reserves of an additional 1.8 million tons within their claim.
In event of a thorium fuel cycle, Conway granite with 56 (±6) parts per million thorium could provide a major low-grade resource; a 307 sq mile (795 sq km) "main mass" in New Hampshire is estimated to contain over three million metric tons per 100 feet (30 m) of depth ( i.e. 1 kg thorium in eight cubic metres of rock), of which two-thirds is "readily leachable". Even common granite rock with 13 PPM thorium concentration (just twice the crustal average, along with 4 ppm uranium) contains potential nuclear energy equivalent to 50 times the entire rock's mass in coal, although there is no incentive to resort to such very low-grade deposits so long as much higher-grade deposits remain available and cheaper to extract. Thorium has been produced in excess of demand from the refining of rare earth elements.
Dangers and biological roles
Powdered thorium metal is pyrophoric and will often ignite spontaneously in air. Natural thorium decays very slowly compared to many other radioactive materials, and the alpha radiation emitted cannot penetrate human skin meaning owning and handling small amounts of thorium, such as a gas mantle, is considered safe. Exposure to an aerosol of thorium, however, can lead to increased risk of cancers of the lung, pancreas, and blood, as lungs and other internal organs can be penetrated by alpha radiation. Exposure to thorium internally leads to increased risk of liver diseases. Thorium is radioactive and produces a radioactive gas, radon-220, as one of its decay products. Secondary decay products of thorium include radium and actinium. Because of this, there are concerns about the safety of thorium mantles. Some nuclear safety agencies make recommendations about their use. Production of gas mantles has led to some safety concerns during manufacture.
The element has no known biological role. Humans typically consume three micrograms per day of thorium. Of this, 99.98% does not remain in the body. Out of the thorium that does remain in the body, three quarters of it accumulates in the skeleton. A number of thorium compounds are chemically moderately toxic. People who work with thorium compounds are at a risk of dermatitis. Exposure to thorium over a long time can cause cancer. It can take as much as thirty years after the ingestion of thorium for symptoms to manifest themselves.