Astatine
About this schools Wikipedia selection
This Wikipedia selection is available offline from SOS Children for distribution in the developing world. Do you want to know about sponsoring? See www.sponsorachild.org.uk
| Astatine | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
85At
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Appearance | ||||||||||||||||||||||||||||||||||
| unknown, but probably a black solid; possibly metallic in appearance | ||||||||||||||||||||||||||||||||||
| General properties | ||||||||||||||||||||||||||||||||||
| Name, symbol, number | astatine, At, 85 | |||||||||||||||||||||||||||||||||
| Pronunciation | / ˈ æ s t ə t iː n / AS-tə-teen or / ˈ æ s t ə t ɪ n / AS-tə-tin |
|||||||||||||||||||||||||||||||||
| Metallic category | halogen sometimes classified as a metalloid (disputed) |
|||||||||||||||||||||||||||||||||
| Group, period, block | 17 (halogens), 6, p | |||||||||||||||||||||||||||||||||
| Standard atomic weight | (210) | |||||||||||||||||||||||||||||||||
| Electron configuration | [Xe] 4f14 5d10 6s2 6p5 2, 8, 18, 32, 18, 7 |
|||||||||||||||||||||||||||||||||
| History | ||||||||||||||||||||||||||||||||||
| Discovery | Dale R. Corson, Kenneth Ross MacKenzie, Emilio Segrè (1940) | |||||||||||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||||||||||
| Phase | solid | |||||||||||||||||||||||||||||||||
| Melting point | 575 K, 302 °C, 576 °F | |||||||||||||||||||||||||||||||||
| Boiling point | 610 K, 337 °C, 639 °F | |||||||||||||||||||||||||||||||||
| Heat of vaporization | (At2) 54.39 kJ·mol−1 | |||||||||||||||||||||||||||||||||
| Vapor pressure | ||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||||||||
| Oxidation states | −1, +1, +3, +5, +7 | |||||||||||||||||||||||||||||||||
| Electronegativity | 2.2 (Pauling scale) | |||||||||||||||||||||||||||||||||
| Ionization energies | 1st: 887.7±38.59 kJ·mol−1 | |||||||||||||||||||||||||||||||||
| Covalent radius | 150 pm | |||||||||||||||||||||||||||||||||
| Van der Waals radius | 202 pm | |||||||||||||||||||||||||||||||||
| Miscellanea | ||||||||||||||||||||||||||||||||||
| Magnetic ordering | no data | |||||||||||||||||||||||||||||||||
| Thermal conductivity | 1.7 W·m−1·K−1 | |||||||||||||||||||||||||||||||||
| CAS registry number | 7440-68-8 | |||||||||||||||||||||||||||||||||
| Most stable isotopes | ||||||||||||||||||||||||||||||||||
| Main article: Isotopes of astatine | ||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
Astatine is a radioactive chemical element with the chemical symbol At and atomic number 85. It occurs on Earth only as the result of the radioactive decay of certain heavier elements. All of its isotopes are short-lived; the most stable is astatine-210, with a half-life of 8.1 hours. Accordingly, much less is known about astatine than most other elements. The observed properties are consistent with it being a heavier analog of iodine; many other properties have been estimated based on this resemblance.
Elemental astatine has never been viewed, because a mass large enough to be seen (by the naked human eye) would be immediately vaporized by the heat generated by its own radioactivity. Astatine may be dark, or it may have a metallic appearance and be a semiconductor, or it may even be a metal. It is likely to have a much higher melting point than does iodine, on par with those of bismuth and polonium. Chemically, astatine behaves more or less as a halogen, being expected to form ionic astatides with alkali or alkaline earth metals; it is known to form covalent compounds with nonmetals, including other halogens. It does, however, also have a notable cationic chemistry that distinguishes it from the lighter halogens. The second longest-lived isotope of astatine, astatine-211, is the only one currently having any commercial application, being employed in medicine to diagnose and treat some diseases via its emission of alpha particles (helium-4 nuclei). Only extremely small quantities are used, however, due to its intense radioactivity.
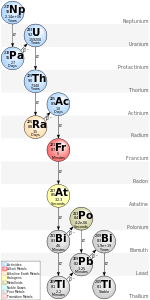
The element was first produced by Dale R. Corson, Kenneth Ross MacKenzie, and Emilio Segrè at the University of California, Berkeley in 1940. They named the element "astatine", a name coming from the great instability of the synthesized matter (the source Greek word αστατος (astatos) means "unstable"). Three years later it was found in nature, although it is the least abundant element in the Earth's crust among the non- transuranic elements, with an estimated total amount of less than 28 grams (1 oz) at any given time. Six astatine isotopes, with mass numbers of 214 to 219, are present in nature as the products of various decay routes of heavier elements, but neither the most stable isotope of astatine (with mass number 210) nor astatine-211 (which is used in medicine) is produced naturally.
Characteristics
Astatine is an extremely radioactive element; all its isotopes have half-lives of less than 12 hours, decaying into bismuth, polonium, radon, or other astatine isotopes. Among the first 101 elements in the periodic table, only francium is less stable.
The bulk properties of astatine are not known with any great degree of certainty. Research is limited by its short half-life, which prevents the creation of weighable quantities. A visible piece of astatine would be immediately and completely vaporized due to the heat generated by its intense radioactivity. Astatine is usually classified as either a nonmetal or a metalloid. However, metal formation for condensed-phase astatine has also been suggested.
Physical
Most of the physical properties of astatine have been estimated (by interpolation or extrapolation), using various theoretically-grounded or empirically-derived methods. As an example, heavier halogens are darker than are halogens of lesser atomic weight – fluorine is nearly colorless, chlorine is bright green, bromine is brown, and iodine is dark gray/violet. Astatine is sometimes described as being a black solid (assuming that it follows this trend), or as having a metallic appearance (if it is a metalloid or a metal). The melting and boiling points of astatine are also expected to follow the trend seen in the halogen series, increasing with atomic number. On this basis, the melting and boiling points are estimated to be 575 K (302 °C; 575 °F) and 610 K (337 °C; 638 °F), respectively. However, some experimental evidence suggests astatine may have lower melting and boiling points than those implied by the halogen trend. Astatine sublimes less readily than does iodine, having a lower vapor pressure. Even so, half of a given amount of astatine will vaporize in an hour if put on a clean glass surface at room temperature.
The crystalline structure of solid astatine is unknown. Evidence for (or against) the existence of diatomic astatine (At2) is sparse and inconclusive. Some sources state that At2 does not exist, or at least has never been observed, while other sources assert or imply its existence. Despite this controversy, many properties of diatomic astatine have been predicted.
Chemical
Many chemical properties of astatine have been determined using tracer studies on extremely dilute astatine solutions. Most known properties – such as anion formation – are in line with other halogens. However, astatine has a few metallic characteristics as well, such as plating out on a cathode, coprecipitating with metal sulfides in hydrochloric acid, and forming a cation in strong acidic solutions.
Astatine has an electronegativity of 2.2 on the revised Pauling scale. This is lower than that of iodine (2.66) and the same as that of hydrogen. However, in hydrogen astatide (HAt) the negative charge is predicted to be on the hydrogen atom, implying that this compound should instead be referred to as astatine hydride. In this context, it is pertinent to note that the electronegativity of astatine on the Allred-Rochow scale (1.9) is less than that of hydrogen (2.2).
Compounds
Astatine is the least reactive of the halogens, being less reactive than iodine; however, multiple compounds of astatine have been synthesized in microscopic amounts and studied as intensively as possible before their inevitable radioactive disintegration. The reactions involved are normally tested with dilute solutions of astatine mixed with larger amounts of iodine. The iodine acts as a carrier, ensuring that there is sufficient material for laboratory techniques (such as filtration and precipitation) to work.
The formation of an astatine compound with hydrogen – usually referred to as hydrogen astatide – was noted by the pioneers of astatine chemistry. As mentioned earlier, there are grounds for referring to this compound as astatine hydride instead – astatine is easily oxidized, acidification by (dilute) nitric acid gives the At0 or At+ forms, and the addition of silver(I) then precipitates astatine, only partially as silver(I) astatide (AgAt) (or not at all). Iodine, in contrast, is not oxidized, and precipitates readily as silver(I) iodide.
Only a few metal astatides have been reported, including those of sodium, palladium, silver, and lead. Some characteristic properties of silver astatide, and the known and hypothetical alkali and alkaline earth astatides, have been estimated by extrapolation from other silver or alkali or alkaline earth halides.
Astatine is known to react with its lighter homologues iodine, bromine, and chlorine in the vapor state; these reactions produce diatomic interhalogen compounds with formulas AtI, AtBr, and AtCl. The first two compounds may also be produced in water – astatine reacts with iodine/ iodide solution to form AtI, whereas AtBr requires (aside from astatine) an iodine/ iodine monobromide/ bromide solution. The excess of iodides or bromides may lead to AtBr−
2 and AtI−
2 ions, or in a chloride solution, they may produce species like AtCl−
2 or AtBrCl− via equilibrium-balanced reactions with the chlorides. Oxidation of the element with dichromate (in nitric acid solution) showed that adding chloride turned the astatine into a molecule likely to be either AtCl or AtOCl. Similarly, AtOCl−
2 or AtCl−
2 may be produced. In a plasma ion source mass spectrometer, the similar ions [AtI]+, [AtBr]+, and [AtCl]+ have been formed by introducing lighter halogen vapors into a helium-filled cell containing astatine, supporting the existence of stable neutral molecules in the plasma ion state. No astatine fluorides have been discovered as yet. Their absence has been speculatively attributed to the extreme reactivity of such compounds, including the reaction of an initially-formed fluoride with the walls of the glass container to form a non-volatile product. Thus, although the synthesis of an astatine fluoride is thought to be possible, it may require a liquid halogen fluoride solvent, as has already been used for the characterization of radon fluorides.
With oxygen, there is evidence for the existence of the species AtO–, AtO−
2 and AtO+ in aqueous solution, formed by the reaction of astatine with an oxidant such as elemental bromine or (in the last case) by sodium persulfate in a solution of perchloric acid. The well characterized AtO−
3 anion can be obtained by, for example, the oxidation of astatine with potassium hypochlorite in a solution of potassium hydroxide. Further oxidation, such as by xenon difluoride (in a hot alkaline solution) or periodate (in a neutral or alkaline solution), yields the perastatate ion AtO−
4; however, this is only stable in neutral or alkaline solutions. Astatine is also thought to be capable of forming cationic salts with oxyanions such as iodate or dichromate; this is based on the observation that, in acidic solutions, monovalent or intermediate positive states of astatine coprecipitate with the insoluble salts of metal cations such as silver(I) iodate or thallium(I) dichromate.
Astatine may form bonds to the other chalcogens; these include S7At+ and At(CSN)−
2 with sulfur, a coordination selenourea compound with selenium, and an astatine–tellurium colloid with tellurium. Additionally, astatine is known to bind to nitrogen, lead, and boron under the proper conditions.
Carbon tetraastatide (CAt4) is known. Astatine can replace a hydrogen atom in benzene to form C6H5At; this may be oxidized to C6H5AtCl2 by chlorine. By treating this compound with an alkaline solution of hypochlorite, C6H5AtO2 can be produced.
History
In 1869, when Dmitri Mendeleev published his periodic table, the space under iodine was empty; after Niels Bohr established the physical basis of the classification of chemical elements, it was suggested that the fifth halogen belonged there. Before its officially recognized discovery, it was called "eka-iodine" (from Sanskrit eka – "one") to imply it was one space under iodine (in the same manner as eka-silicon, eka-boron, and others). Scientists tried to find it in nature; given its rarity, these attempts resulted in a number of false discoveries.
The first claimed discovery of eka-iodine was made by Fred Allison and his associates at the Alabama Polytechnic Institute (now Auburn University) in 1931. The discoverers named element 85 "alabamine", and assigned it the symbol Ab, designations that were used for a few years afterward. In 1934, however, H. G. MacPherson of University of California, Berkeley disproved Allison's method and the validity of his discovery. This erroneous discovery was followed by another claim in 1937, by the chemist Rajendralal De. Working in Dhaka, British India (now Bangladesh), he chose the name "dakin" for element 85, which he claimed to have isolated as the thorium series equivalent of Radium F (polonium-210) in the radium series. The properties he reported for dakin do not correspond to those of astatine, and the true identity of dakin is not known.
In 1940, the Swiss chemist Walter Minder announced the discovery of element 85 as the beta decay product of Radium A (polonium-218), choosing the name "helvetium" (from Helvetia, "Switzerland"). However, Berta Karlik and Traude Bernert were unsuccessful in reproducing his experiments, and subsequently attributed Minder's results to contamination of his radon stream (radon-222 is the parent isotope of polonium-218). In 1942, Minder, in collaboration with the English scientist Alice Leigh-Smith, announced the discovery of another isotope of element 85, presumed to be the product of Thorium A (polonium-216) beta decay. They named this substance "anglo-helvetium", but Karlik and Bernert were again unable to reproduce these results.
In 1940, Dale R. Corson, Kenneth Ross MacKenzie, and Emilio Segrè finally isolated the element at the University of California, Berkeley. Instead of searching for the element in nature, the scientists created it by bombarding bismuth-209 with alpha particles. The name "astatine" comes from the Greek word αστατος (astatos, meaning "unstable"), due to its propensity for radioactive decay (later, all isotopes of the element were shown to be unstable), together with the ending "-ine", found in the names of the four previously discovered halogens. Three years later, astatine was found as a product of naturally occurring decay chains by Karlik and Bernert. Since then, astatine has been determined to be in three out of the four natural decay chains.
Isotopes
There are 32 known isotopes of astatine, with atomic masses (mass numbers) of 191 and 193–223. No stable or even long-lived astatine isotope is known, and no such isotope is expected to exist.
-
Alpha decay characteristics for sample astatine isotopes Mass
numberMass
excessMass
excess of
daughterAverage
energy of
alpha
decayHalf-life Probability
of alpha
decayAlpha
half-life207 −13.243 MeV −19.116 MeV 5.873 MeV 1.80 h 8.6% 20.9 h 208 −12.491 MeV −18.243 MeV 5.752 MeV 1.63 h 0.55% 12.3 d 209 −12.880 MeV −18.638 MeV 5.758 MeV 5.41 h 4.1% 5.5 d 210 −11.972 MeV −17.604 MeV 5.632 MeV 8.1 h 0.175% 193 d 211 −11.647 MeV −17.630 MeV 5.983 MeV 7.21 h 41.8% 17.2 h 212 −8.621 MeV −16.436 MeV 7.825 MeV 0.31 s ≈100% 0.31 s 213 −6.579 MeV −15.834 MeV 9.255 MeV 125 ns 100% 125 ns 214 −3.380 MeV −12.366 MeV 8.986 MeV 558 ns 100% 558 ns 219 10.397 MeV 4.073 MeV 6.324 MeV 56 s 97% 58 s 220 14.350 MeV 8.298 MeV 6.052 MeV 3.71 min 8% 46.4 min 221 16.810 MeV 11.244 MeV 5.566 MeV 2.3 min experimentally
alpha stable∞
Astatine has 23 nuclear isomers, which are nuclei with one or more nucleons (protons or neutrons) in an excited state. A nuclear isomer may also be called a " meta-state", meaning the system has more internal energy than the " ground state" (the state with the lowest possible internal energy), making the former likely to decay into the latter. There may be more than one isomer for each isotope. The most stable of these nuclear isomers is astatine-202m1, which has a half-life of about 3 minutes, longer than those of all the ground states except for those of isotopes 203–211 and 220. The least stable one is astatine-214m1; its half-life of 265 nanoseconds is shorter than those of all ground states except that of astatine-213.
Astatine's alpha decay energies follow the same trend as for other heavy elements. Lighter astatine isotopes have quite high energies of alpha decay, which become lower as the nuclei become heavier. Astatine-211, however, has a significantly higher energy than the previous isotope, because it has a nucleus with 126 neutrons, and 126 is a magic number corresponding to a filled neutron shell. Despite having a similar half-life to the previous isotope (8.1 hours for astatine-210 and 7.2 hours for astatine-211), the alpha decay probability is much higher for the latter: 41.81% against only 0.18%. The two following isotopes release even more energy, with astatine-213 releasing the highest amount of energy of all astatine isotopes. For this reason, it is the shortest-lived astatine isotope. Even though heavier astatine isotopes release less energy, no long-lived astatine isotope exists, due to the increasing role of beta decay (electron emission). This decay mode is especially important for astatine; as early as 1950 it was postulated that the element has no beta-stable isotopes (i.e., ones that do not beta decay at all). Beta decay modes have been found for all astatine isotopes except astatine-213, astatine-214, astatine-215, and astatine-216m. Astatine-210 and lighter isotopes exhibit beta plus decay ( positron emission), astatine-216 and heavier isotopes exhibit beta (minus) decay, and astatine-212 decays via both modes, while astatine-211 undergoes electron capture instead.
The most stable isotope is astatine-210, which has a half-life of 8.1 hours. This isotope's primary decay mode is beta plus decay to the relatively long-lived (in comparison to astatine isotopes) alpha emitter polonium-210. In total, only five isotopes have half-lives exceeding one hour (those with mass numbers between 207 and 211). The least stable ground state isotope is astatine-213, with a half-life of 125 nanoseconds. It alpha decays to the extremely long-lived (in practice, stable) bismuth-209.
Natural occurrence
Astatine is the rarest naturally occurring element that is not a transuranic element, with the total amount in Earth's crust estimated to be less than 28 grams (1 oz) at any given time. Any astatine that was present at the Earth's formation has long since decayed, and the minute amounts of astatine existing currently have formed through the decay of heavier elements. While it was previously thought to be the rarest element occurring on the Earth, astatine has lost this status to berkelium, atoms of which can be produced by neutron capture reactions and beta decay in very highly concentrated uranium-bearing deposits.
Six astatine isotopes occur naturally (astatine-214 to astatine-219). Because of their short half-lives, they are found only in trace amounts. There is no data indicating that astatine occurs in stars.
Four out of these isotopes (astatine-215, astatine-217, astatine-218, and astatine-219) are found due to their production in major natural decay chains. Francium-223, the father isotope of astatine-219, alpha decays with a probability of only 0.006%, making this astatine isotope extremely rare even compared to the other astatine isotopes; this is in spite of its half-life being the longest of the natural astatine isotopes (56 seconds). Astatine-219 decays to polonium-215, which itself beta decays to astatine-215 with an even smaller probability of 0.00023%. The entirety of North and South America combined, considered to a depth of 16 kilometers (10 miles), contain only about one trillion astatine-215 atoms at any given time. Astatine-218 is found in nature as a result of polonium-218 beta decay; as with francium-223 and polonium-215, decay to an astatine isotope is not the primary decay mode. However, the astatine-217 isotope has a straight chain leading directly to astatine; its father isotope (francium-221) decays exclusively to this nuclide. Given that its fathers, grandfathers, and so on each decay exclusively to only one nuclide, this gives only one possible way for the starting nuclide in the neptunium series (neptunium-237) to decay – via eventual production of astatine-217.
The isotopes with mass numbers 214 through 216 are found as the result of triple alpha decay of the naturally present protactinium isotopes protactinium-226, protactinium-227, and protactinium-228. However, these isotopes are extremely rare, so much so that they are often not cited as natural astatine isotopes.
Synthesis
Formation
-
Possible reactions after bombarding bismuth-209 with alpha particles Reaction Energy of alpha particle 209
83Bi + 4
2He → 211
85At + 2 1
026 MeV 209
83Bi + 4
2He → 210
85At + 3 1
040 MeV 209
83Bi + 4
2He → 209
85At + 4 1
060 MeV
Astatine was first produced by bombarding bismuth-209 with energetic alpha particles, and this is still the major route used to create the relatively long-lived isotopes astatine-209 through astatine-211. Astatine is only produced in microscopic quantities, with modern techniques allowing production runs of 2 tera becquerels (about 25 micrograms).
The most important isotope is astatine-211, the only one to currently have a commercial use. To produce the bismuth target, the metal is sputtered onto a gold, copper, or aluminium surface at 50 to 100 milligrams per square centimeter. The bismuth layer, or alternatively bismuth oxide, is forcibly fused with a copper plate. The target is kept under a chemically neutral nitrogen atmosphere, and is cooled with water to prevent premature astatine vaporization. In a particle accelerator, such as a cyclotron, alpha particles (helium-4 nuclei) are collided with the bismuth. Even though there is only one bismuth isotope used (bismuth-209), the reaction may occur in three possible ways, producing astatine-209, astatine-210, or astatine-211. In order to eliminate undesired nuclides, the maximum energy of the particle accelerator is set to any value (such as 30 MeV) above that for the reaction producing astatine-211 (to produce the desired isotope) and below the one producing astatine-210 (to avoid producing other astatine isotopes).
Separation
Since the element is the main product of the synthesis, after its formation it must only be separated from the target and traces of other radioisotopes. The astatine-containing target is heated to 270 °C (520 °F) to vaporize away volatile traces of radioisotopes, after which the temperature is raised to 800 °C (1450 °F). Although 80% of astatine may vaporize at this temperature, bismuth begins to vaporize as well. Astatine's vaporization does not occur at an adequate rate at temperatures below 600 °C (1100 °F), but at temperatures above 800 °C (1450 °F), astatine's volatility from a bismuth surface increases significantly. The condensed vapor (distillate) is collected on a water-cooled platinum surface, which is later moved into a U-like quartz vessel. The quartz container vessel is heated to 130 °C (270 °F) to remove further traces of impurities (typically polonium) and then to 500 °C (930 °F) to remove astatine, which is collected on a cold finger. The purified element is then washed off the cold finger with a weak nitric acid solution. Using this technique, yields of astatine of up to 30% may be achieved.
Uses and precautions
-
Several 211At-containing molecules and their uses Agent Applications [211At]astatine-tellurium colloids Compartmental tumors 6-[211At]astato-2-methyl-1,4-naphtaquinol diphosphate Adenocarcinomas 211At-labeled methylene blue Melanomas Meta-[211At]astatobenzyl guanidine Neuroendocrine tumors 5-[211At]astato-2'-deoxyuridine Various 211At-labeled biotin conjugates Various pretargeting 211At-labeled octreotide Somatostatin receptor 211At-labeled mAbs and fragments Various 211At-labeled bisphosphonates Bone metastases
The newly formed astatine-211 is important in nuclear medicine. Once produced, astatine must be used quickly, as it decays with a half-life of 7.2 hours; this is, however, long enough to permit multi-step labeling strategies. Astatine-211 can be used for targeted alpha particle radiotherapy, since it decays either via emission of an alpha particle (to bismuth-207), or via electron capture (to an extremely short-lived nuclide of polonium-211, which itself undergoes further alpha decay).
In a manner similar to iodine, astatine is preferentially concentrated in the thyroid gland, although to a lesser extent. However, it tends to concentrate in the liver in the form of a radiocolloid if it is released into the systemic circulation. The principal medicinal difference between astatine-211 and iodine-131 (a radioactive iodine isotope also used in medicine) is that astatine does not emit high energy beta particles (electrons), as does iodine-131. Beta particles have considerably greater penetrating power through tissues than do the much heavier alpha particles. While an average energy alpha particle released by decay of astatine-211 can travel up to 70 µm through the surrounding tissues, an average energy beta particle emitted by iodine-131 can travel nearly 30 times as far, to about 2 mm. Thus, using astatine-211 instead of iodine-131 enables the thyroid to be dosed appropriately, while the neighboring parathyroid gland is spared. The short half-life and limited penetrating power of its radiation through tissues renders astatine generally preferable to iodine-131 when used in diagnosis as well.
Experiments in rats and monkeys, however, suggest that astatine causes much greater damage to the thyroid gland than does iodine-131, with repetitive injection of the nuclide resulting in necrosis and cell dysplasia within the gland. These experiments also suggest that astatine could cause damage to the thyroid of any organism. Early research suggested that injection of lethal quantities of astatine caused morphological changes in breast tissue (although not other tissues); however, this conclusion currently remains controversial.