Dubnium
About this schools Wikipedia selection
This Schools selection was originally chosen by SOS Children for schools in the developing world without internet access. It is available as a intranet download. Sponsor a child to make a real difference.
| Dubnium | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
105Db
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| unknown | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name, symbol, number | dubnium, Db, 105 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pronunciation | / ˈ d uː b n i ə m / DOOB-nee-əm |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Element category | transition metal | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group, period, block | 5, 7, d | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight | [268] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Rn] 5f14 6d3 7s2 (predicted) 2, 8, 18, 32, 32, 11, 2 (predicted) |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Discovery | Joint Institute for Nuclear Research (1968) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase | solid (predicted) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | 29 (predicted) g·cm−3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | 5, 4, 3 (predicted) (only bolded oxidation states are known experimentally) |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies ( more) |
1st: 664.8 (estimated) kJ·mol−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2nd: 1546.7 (estimated) kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3rd: 2378.4 (estimated) kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | 139 (estimated) pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 149 (estimated) pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Miscellanea | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS registry number | 53850-35-4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Most stable isotopes | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Main article: Isotopes of dubnium | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Dubnium is a chemical element with the symbol Db and atomic number 105, named after the town of Dubna in Russia, where it was first produced. It is a synthetic element (an element that can be created in a laboratory but is not found in nature) and radioactive; the most stable known isotope, dubnium-268, has a half-life of approximately 28 hours.
In the periodic table of the elements, it is a d-block element and in the transactinide elements. It is a member of the 7th period and belongs to the group 5 element. Chemistry experiments have confirmed that dubnium behaves as the heavier homologue to tantalum in group 5. The chemical properties of dubnium are characterized only partly. They are similar with those of other group 5 elements.
In the 1960s, microscopic amounts of dubnium were produced in laboratories in the former Soviet Union and in California. The priority of the discovery and therefore the naming of the element was disputed between Soviet and American scientists, and it was not until 1997 that International Union of Pure and Applied Chemistry (IUPAC) established dubnium as the official name for the element.
History
Discovery
Dubnium was reportedly first discovered in 1968 at the Joint Institute for Nuclear Research at Dubna (then in the Soviet Union). Researchers there bombarded an americium-243 target with neon-22 ions. They reported a 9.40 MeV and a 9.70 MeV alpha-activity and assigned the decays to the isotope 260Db or 261Db:
- 243
95Am + 22
10Ne → 265−x
105Db + x n
Two years later the Dubna team separated their reaction products by thermal gradient chromatography after conversion to chlorides by interaction with NbCl5. The team identified a 2.2 second spontaneous fission activity contained within a volatile chloride portraying eka-tantalum properties, likely dubnium-261 pentachloride, 261DbCl5.
In the same year, a team led by Albert Ghiorso working at the University of California, Berkeley conclusively synthesized the element by bombarding a californium-249 target with nitrogen-15 ions. The team published a convincing synthesis of 260Db in the reaction between californium-249 target and nitrogen-15 ions and measured the alpha decay of 260Db with a half-life of 1.6 seconds and a decay energy of 9.10 MeV, correlated with the daughter decay of lawrencium-256:
- 249
98Cf + 15
7N → 260
105Db + 4 n
These results by the Berkeley scientists did not confirm the Soviet findings regarding the 9.40 MeV or 9.70 MeV alpha-decay of dubnium-260, leaving only dubnium-261 as possible produced isotope. In 1971, the Dubna team repeated their reaction using an improved set-up and were able to confirm the decay data for 260Db using the reaction:
- 243
95Am + 22
10Ne → 260
105Db + 5 n
In 1976, the Dubna team continued their study of the reaction using thermal gradient chromatography and were able to identify the product as dubnium-260 pentabromide, 260DbBr5.
In 1992 the IUPAC/ IUPAP Transfermium Working Group assessed the claims of the two groups and concluded that confidence in the discovery grew from results from both laboratories and the claim of discovery should be shared.
Naming controversy
The Soviet, later Russian, team proposed the name nielsbohrium (Ns) in honour of the Danish nuclear physicist Niels Bohr. The American team proposed that the new element should be named hahnium (Ha), in honour of the late German chemist Otto Hahn. Consequently hahnium was the name that most American and Western European scientists used and appears in many papers published at the time, and nielsbohrium was used in the Soviet Union and Eastern Bloc countries.
An element naming controversy erupted between the two groups. The International Union of Pure and Applied Chemistry (IUPAC) thus adopted unnilpentium (Unp) as a temporary, systematic element name. Attempting to resolve the issue, in 1994, the IUPAC proposed the name joliotium (Jl), after the French physicist Frédéric Joliot-Curie, which was originally proposed by Soviet team for element 102, later named nobelium. The two principal claimants still disagreed about the names of elements 104-106. However, in 1997 they resolved the dispute and adopted the current name, dubnium (Db), after the Russian town of Dubna, the location of the Joint Institute for Nuclear Research. It was argued by IUPAC that the Berkeley laboratory had already been recognized several times in the naming of elements (i.e., berkelium, californium, americium) and that the acceptance of the names rutherfordium and seaborgium for elements 104 and 106 should be offset by recognizing the Russian team's contributions to the discovery of elements 104, 105 and 106.
Chemical properties
Extrapolated properties
Element 105 is projected to be the third member of the 6d series of transition metals and the heaviest member of group V in the Periodic Table, below vanadium, niobium and tantalum. Because it is positioned right below tantalum, it may also be called eka-tantalum. All the members of the group readily portray their oxidation state of +5 and the state becomes more stable as the group is descended. Thus dubnium is expected to form a stable +5 state. For this group, +4 and +3 states are also known for the heavier members and dubnium may also form these reducing oxidation states.
In an extrapolation of the chemistries from niobium and tantalum, dubnium should react with oxygen to form an inert pentoxide, Db2O5. In alkali, the formation of an orthodubnate complex, DbO3−
4, is expected. Reaction with the halogens should readily form the pentahalides, DbX5. The pentachlorides of niobium and tantalum exist as volatile solids or monomeric trigonal bipyramidal molecules in the vapour phase. Thus, DbCl5 is expected to be a volatile solid. Similarly, the pentafluoride, DbF5, should be even more volatile. Hydrolysis of the halides is known to readily form the oxyhalides, MOX3. Thus the halides DbX5 should react with water to form DbOX3. The reaction with fluoride ion is also well known for the lighter homologues and dubnium is expected to form a range of fluoro-complexes. In particular, reaction of the pentafluoride with HF should form a hexafluorodubnate ion, DbF−
6. Excess fluoride should lead to DbF2−
7 and DbOF2−
5. If eka-tantalum properties are portrayed, higher concentrations of fluoride should ultimately form DbF3−
8 since NbF3−
8 is not known.
Experimental chemistry
The chemistry of dubnium has been studied for several years using gas thermochromatography. The experiments have studied the relative adsorption characteristics of isotopes of niobium, tantalum and dubnium radioisotopes. The results have indicated the formation of typical group 5 halides and oxyhalides, namely DbCl5, DbBr5, DbOCl3 and DbOBr3. Reports on these early experiments usually refer to dubnium as hahnium.
| Formula | Names(s) |
|---|---|
| DbCl5 | dubnium pentachloride ; dubnium(V) chloride |
| DbBr5 | dubnium pentabromide ; dubnium(V) bromide |
| DbOCl3 | dubnium oxychloride ; dubnium(V) trichloride oxide ; dubnyl(V) chloride |
| DbOBr3 | dubnium oxybromide ; dubnium(V) tribromide oxide ; dubnyl(V) bromide |
Nucleosynthesis history
Cold fusion
This section deals with the synthesis of nuclei of dubnium by so-called "cold" fusion reactions. These are processes which create compound nuclei at low excitation energy (~10-20 MeV, hence "cold"), leading to a higher probability of survival from fission. The excited nucleus then decays to the ground state via the emission of one or two neutrons only.
- 209Bi(50Ti,xn)259-xDb (x=1,2,3)
The first attempts to synthesise dubnium using cold fusion reactions were performed in 1976 by the team at FLNR, Dubna using the above reaction. They were able to detect a 5 s spontaneous fission (SF) activity which they assigned to 257Db. This assignment was later corrected to 258Db. In 1981, the team at GSI studied this reaction using the improved technique of correlation of genetic parent-daughter decays. They were able to positively identify 258Db, the product from the 1n neutron evaporation channel. In 1983, the team at Dubna revisited the reaction using the method of identification of a descendant using chemical separation. They succeeded in measuring alpha decays from known descendants of the decay chain beginning with 258Db. This was taken as providing some evidence for the formation of dubnium nuclei. The team at GSI revisited the reaction in 1985 and were able to detect 10 atoms of 257Db. After a significant upgrade of their facilities in 1993, in 2000 the team measured 120 decays of 257Db, 16 decays of 256Db and decay of 258Db in the measurement of the 1n, 2n and 3n excitation functions. The data gathered for 257Db allowed a first spectroscopic study of this isotope and identified an isomer, 257mDb, and a first determination of a decay level structure for 257Db. The reaction was used in spectroscopic studies of isotopes of mendelevium and einsteinium in 2003–2004.
- 209Bi(49Ti,xn)258-xDb (x=2?)
This reaction was studied by Yuri Oganessian and the team at Dubna in 1983. They observed a 2.6 s SF activity tentatively assigned to 256Db. Later results suggest a possible reassignment to 256Rf, resulting from the ~30% EC branch in 256Db.
- 209Bi(48Ti,xn)257-xDb (x=1?)
This reaction was studied by Yuri Oganessian and the team at Dubna in 1983. They observed a 1.6 s activity with a ~80% alpha branch with a ~20% SF branch. The activity was tentatively assigned to 255Db. Later results suggest a reassignment to 256Db.
- 208Pb(51V,xn)259-xDb (x=1,2)
The team at Dubna also studied this reaction in 1976 and were again able to detect the 5 s SF activity, first tentatively assigned to 257Db and later to 258Db. In 2006, the team at LBNL reinvestigated this reaction as part of their odd-Z projectile program. They were able to detect 258Db and 257Db in their measurement of the 1n and 2n neutron evaporation channels.
- 207Pb(51V,xn)258-xDb
The team at Dubna also studied this reaction in 1976 but this time they were unable to detect the 5 s SF activity, first tentatively assigned to 257Db and later to 258Db. Instead, they were able to measure a 1.5 s SF activity, tentatively assigned to 255Db.
- 205Tl(54Cr,xn)259-xDb (x=1?)
The team at Dubna also studied this reaction in 1976 and were again able to detect the 5 s SF activity, first tentatively assigned to 257Db and later to 258Db.
Hot fusion
This section deals with the synthesis of nuclei of dubnium by so-called "hot" fusion reactions. These are processes which create compound nuclei at high excitation energy (~40-50 MeV, hence "hot"), leading to a reduced probability of survival from fission and quasi-fission. The excited nucleus then decays to the ground state via the emission of 3-5 neutrons.
- 232Th(31P,xn)263-xDb (x=5)
There are very limited reports that this rare reaction using a P-31 beam was studied in 1989 by Andreyev et al. at the FLNR. One source suggests that no atoms were detected whilst a better source from the Russians themselves indicates that 258Db was synthesised in the 5n channel with a yield of 120 pb.
- 238U(27Al,xn)265-xDb (x=4,5)
In 2006, as part of their study of the use of uranium targets in superheavy element synthesis, the LBNL team led by Ken Gregorich studied the excitation functions for the 4n and 5n channels in this new reaction.
- 236U(27Al,xn)263-xDb (x=5,6)
This reaction was first studied by Andreyev et al. at the FLNR, Dubna in 1992. They were able to observe 258Db and 257Db in the 5n and 6n exit channels with yields of 450 pb and 75 pb, respectively.
- 243Am(22Ne,xn)265-xDb (x=5)
The first attempts to synthesise dubnium were performed in 1968 by the team at the Flerov Laboratory of Nuclear Reactions (FLNR) in Dubna, Russia. They observed two alpha lines which they tentatively assigned to 261Db and 260Db. They repeated their experiment in 1970 looking for spontaneous fission. They found a 2.2 s SF activity which they assigned to 261Db. In 1970, the Dubna team began work on using gradient thermochromatography in order to detect dubnium in chemical experiments as a volatile chloride. In their first run they detected a volatile SF activity with similar adsorption properties to NbCl5 and unlike HfCl4. This was taken to indicate the formation of nuclei of dvi-niobium as DbCl5. In 1971, they repeated the chemistry experiment using higher sensitivity and observed alpha decays from an dvi-niobium component, taken to confirm the formation of 260Db. The method was repeated in 1976 using the formation of bromides and obtained almost identical results, indicating the formation of a volatile, dvi-niobium-like DbBr5.
- 241Am(22Ne,xn)263-xDb (x=4,5)
In 2000, Chinese scientists at the Institute of Modern Physics (IMP), Lanzhou, announced the discovery of the previously unknown isotope 259Db formed in the 4n neutron evaporation channel. They were also able to confirm the decay properties for 258Db.
- 248Cm(19F,xn)267-xDb (x=4,5)
This reaction was first studied in 1999 at the Paul Scherrer Institute (PSI) in order to produce 262Db for chemical studies. Just 4 atoms were detected with a cross section of 260 pb. Japanese scientists at JAERI studied the reaction further in 2002 and determined yields for the isotope 262Db during their efforts to study the aqueous chemistry of dubnium.
- 249Bk(18O,xn)267-xDb (x=4,5)
Following from the discovery of 260Db by Albert Ghiorso in 1970 at the University of California (UC), the same team continued in 1971 with the discovery of the new isotope 262Db. They also observed an unassigned 25 s SF activity, probably associated with the now-known SF branch of 263Db. In 1990, a team led by Kratz at LBNL definitively discovered the new isotope 263Db in the 4n neutron evaporation channel. This reaction has been used by the same team on several occasions in order to attempt to confirm an electron capture (EC) branch in 263Db leading to long-lived 263Rf (see rutherfordium).
- 249Bk(16O,xn)265-xDb (x=4)
Following from the discovery of 260Db by Albert Ghiorso in 1970 at the University of California (UC), the same team continued in 1971 with the discovery of the new isotope 261Db.
- 250Cf(15N,xn)265-xDb (x=4)
Following from the discovery of 260Db by Ghiorso in 1970 at LBNL, the same team continued in 1971 with the discovery of the new isotope 261Db.
- 249Cf(15N,xn)264-xDb (x=4)
In 1970, the team at the Lawrence Berkeley National Laboratory (LBNL) studied this reaction and identified the isotope 260Db in their discovery experiment. They used the modern technique of correlation of genetic parent-daughter decays to confirm their assignment. In 1977, the team at Oak Ridge repeated the experiment and were able to confirm the discovery by the identification of K X-rays from the daughter lawrencium.
- 254Es(13C,xn)267-xDb
In 1988, scientists as the Lawrence Livermore National Laboratory (LLNL) used the asymmetric hot fusion reaction with an einsteinium-254 target to search for the new nuclides 264Db and 263Db. Due to the low sensitivity of the experiment caused by the small Es-254 target,they were unable to detect any evaporation residues (ER).
Decay of heavier nuclides
Isotopes of dubnium have also been identified in the decay of heavier elements. Observations to date are summarised in the table below:
| Evaporation Residue | Observed dubnium isotope |
|---|---|
| 294Uus | 270Db |
| 288Uup | 268Db |
| 287Uup | 267Db |
| 282Uut | 266Db |
| 267Bh | 263Db |
| 278Uut, 266Bh | 262Db |
| 265Bh | 261Db |
| 272Rg | 260Db |
| 266Mt, 262Bh | 258Db |
| 261Bh | 257Db |
| 260Bh | 256Db |
Isotopes
| Isotope | Year discovered | discovery reaction |
|---|---|---|
| 256Db | 1983?, 2000 | 209Bi(50Ti,3n) |
| 257Dbg | 1985 | 209Bi(50Ti,2n) |
| 257Dbm | 2000 | 209Bi(50Ti,2n) |
| 258Db | 1976?, 1981 | 209Bi(50Ti,n) |
| 259Db | 2001 | 241Am(22Ne,4n) |
| 260Db | 1970 | 249Cf(15N,4n) |
| 261Db | 1971 | 249Bk(16O,4n) |
| 262Db | 1971 | 249Bk(18O,5n) |
| 263Db | 1971?, 1990 | 249Bk(18O,4n) |
| 264Db | unknown | |
| 265Db | unknown | |
| 266Db | 2006 | 237Np(48Ca,3n) |
| 267Db | 2003 | 243Am(48Ca,4n) |
| 268Db | 2003 | 243Am(48Ca,3n) |
| 269Db | unknown | |
| 270Db | 2009 | 249Bk(48Ca,3n) |
Isomerism
- 260Db
Recent data on the decay of 272Rg has revealed that some decay chains continue through 260Db with extraordinary longer life-times than expected. These decays have been linked to an isomeric level decaying by alpha decay with a half-life of ~19 s. Further research is required to allow a definite assignment.
- 258Db
Evidence for an isomeric state in 258Db has been gathered from the study of the decay of 266Mt and 262Bh. It has been noted that those decays assigned to an electron capture (EC) branch has a significantly different half-life to those decaying by alpha emission. This has been taken to suggest the existence of an isomeric state decaying by EC with a half-life of ~20 s. Further experiments are required to confirm this assignment.
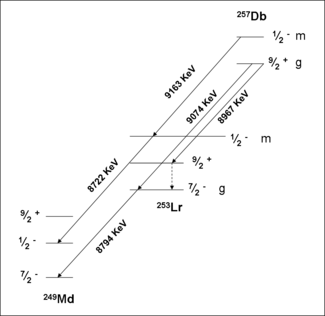
- 257Db
A study of the formation and decay of 257Db has proved the existence of an isomeric state. Initially, 257Db was taken to decay by alpha emission with energies 9.16,9.07 and 8.97 MeV. A measurement of the correlations of these decays with those of 253Lr have shown that the 9.16 MeV decay belongs to a separate isomer. Analysis of the data in conjunction with theory have assigned this activity to a meta stable state, 257mDb. The ground state decays by alpha emission with energies 9.07 and 8.97 MeV. Spontaneous fission of 257m,gDb was not confirmed in recent experiments.
Spectroscopic decay level schemes
- 257Db
Retracted isotopes
- 255Db
In 1983, scientists at Dubna carried out a series of supportive experiments in their quest for the discovery of Bohrium. In two such experiments, they claimed they had detected a ~1.5 s spontaneous fission activity from the reactions 207Pb(51V,xn) and 209Bi(48Ti,xn). The activity was assigned to 255Db. Later research suggested that the assignment should be changed to 256Db. As such, the isotope 255Db is currently not recognised on the chart of radionuclides and further research is required to confirm this isotope.