Quorum sensing is a system of stimulus and response correlated to population density. Many species of bacteria use quorum sensing to coordinate gene expression according to the density of their local population. In similar fashion, some social insects use quorum sensing to determine where to nest. In addition to its function in biological systems, quorum sensing has several useful applications for computing and robotics.
Quorum sensing can function as a decision-making process in any decentralized system, as long as individual components have: (a) a means of assessing the number of other components they interact with and (b) a standard response once a threshold number of components is detected.
Quorum sensing may be achieved by degrading the signalling molecule. Using a KG medium, quorum quenching bacteria can be readily isolated from various environments including that which has previously been considered as unculturable. Recently, a well-studied quorum quenching bacterium has been isolated and its AHL degradation kinetic has been studied by using rapid resolution liquid chromatography (RRLC).
Some of the best-known examples of quorum sensing come from studies of bacteria. Bacteria use quorum sensing to coordinate certain behaviors based on the local density of the bacterial population . Quorum sensing can occur within a single bacterial species as well as between diverse species, and can regulate a host of different processes, in essence, serving as a simple communication network. A variety of different molecules can be used as signals. Common classes of signaling molecules are oligopeptides in Gram-positive bacteria, N-Acyl Homoserine Lactones (AHL) in Gram-negative bacteria, and a family of autoinducers known as autoinducer-2 (AI-2) in both Gram-negative and Gram-positive bacteria.
Quorum Sensing
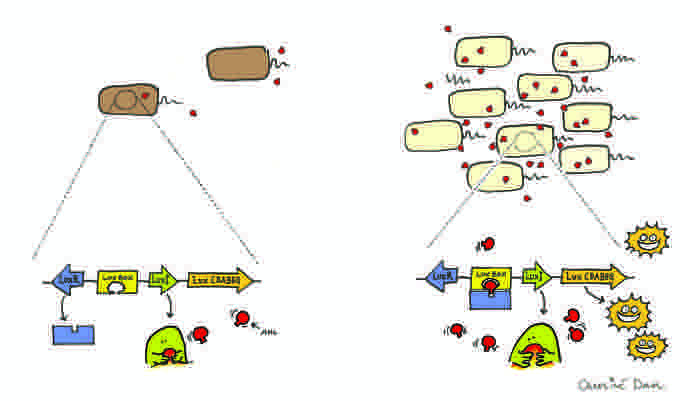
Model of Quorum sensing.
Bacteria that use quorum sensing constitutively produce and secrete certain signaling molecules (called autoinducers or pheromones). These bacteria also have a receptor that can specifically detect the signaling molecule (inducer). When the inducer binds the receptor, it activates transcription of certain genes, including those for inducer synthesis. There is a low likelihood of a bacterium detecting its own secreted inducer. Thus, in order for gene transcription to be activated, the cell must encounter signaling molecules secreted by other cells in its environment. When only a few other bacteria of the same kind are in the vicinity, diffusion reduces the concentration of the inducer in the surrounding medium to almost zero, so the bacteria produce little inducer. However, as the population grows, the concentration of the inducer passes a threshold, causing more inducer to be synthesized. This forms a positive feedback loop, and the receptor becomes fully activated. Activation of the receptor induces the up-regulation of other specific genes, causing all of the cells to begin transcription at approximately the same time. This coordinated behavior of bacterial cells can be useful in a variety of situations. For instance, the bioluminescent luciferase produced by Vibrio fischeri would not be visible if it were produced by a single cell. By using quorum sensing to limit the production of luciferase to situations when cell populations are large, V. fischeri cells are able to avoid wasting energy on the production of useless product.
Three-dimensional structures of proteins involved in quorum sensing were first published in 2001, when the crystal structures of three LuxS orthologs were determined by X-ray crystallography. In 2002, the crystal structure of the receptor LuxP of Vibrio harveyi with its inducer AI-2 (which is one of the few biomolecules containing boron) bound to it was also determined. Many bacterial species, including E. coli, an enteric bacterium and model organism for Gram-negative bacteria, produce AI-2. A comparative genomic and phylogenetic analysis of 138 genomes of bacteria, archaea, and eukaryotes found that "the LuxS enzyme required for AI-2 synthesis is widespread in bacteria, while the periplasmic binding protein LuxP is present only in Vibrio strains," leading to the conclusion that either "other organisms may use components different from the AI-2 signal transduction system of Vibrio strains to sense the signal of AI-2 or they do not have such a quorum sensing system at all. " Certain bacteria can produce enzymes called lactonases that can target and inactivate AHLs.