Two-Component Systems
In molecular biology, two-component systems serve as a basic stimulus-response coupling mechanism allowing organisms to sense and respond to changes in many different environmental conditions. They typically consist of a membrane-bound histidine kinase that senses a specific environmental stimulus and a corresponding response regulator that mediates the cellular response. Two component signaling systems are widely occurring in prokaryotes whereas only a few two-component systems have been identified in eukaryotic organisms.
Signal transduction occurs through the transfer of phosphoryl groups from adenosine triphosphate (ATP) to a specific histidine residue in the histidine kinases (HK). This is an autophosphorylation reaction. Subsequently the histidine kinase catalyzes the transfer of the phosphate group on the phosphorylated histidine residues to an aspartic acid residue on the response regulator (RR). Phosphorylation causes the response regulator's conformation to change, usually activating an attached output domain, which then leads to the stimulation (or repression) of expression of target genes. The level of phosphorylation of the response regulator controls its activity. Some HK are bifunctional, catalysing both the phosphorylation and dephosphorylation of their cognate RR. The input stimuli can regulate either the kinase or phosphatase activity of the bifunctional HK.
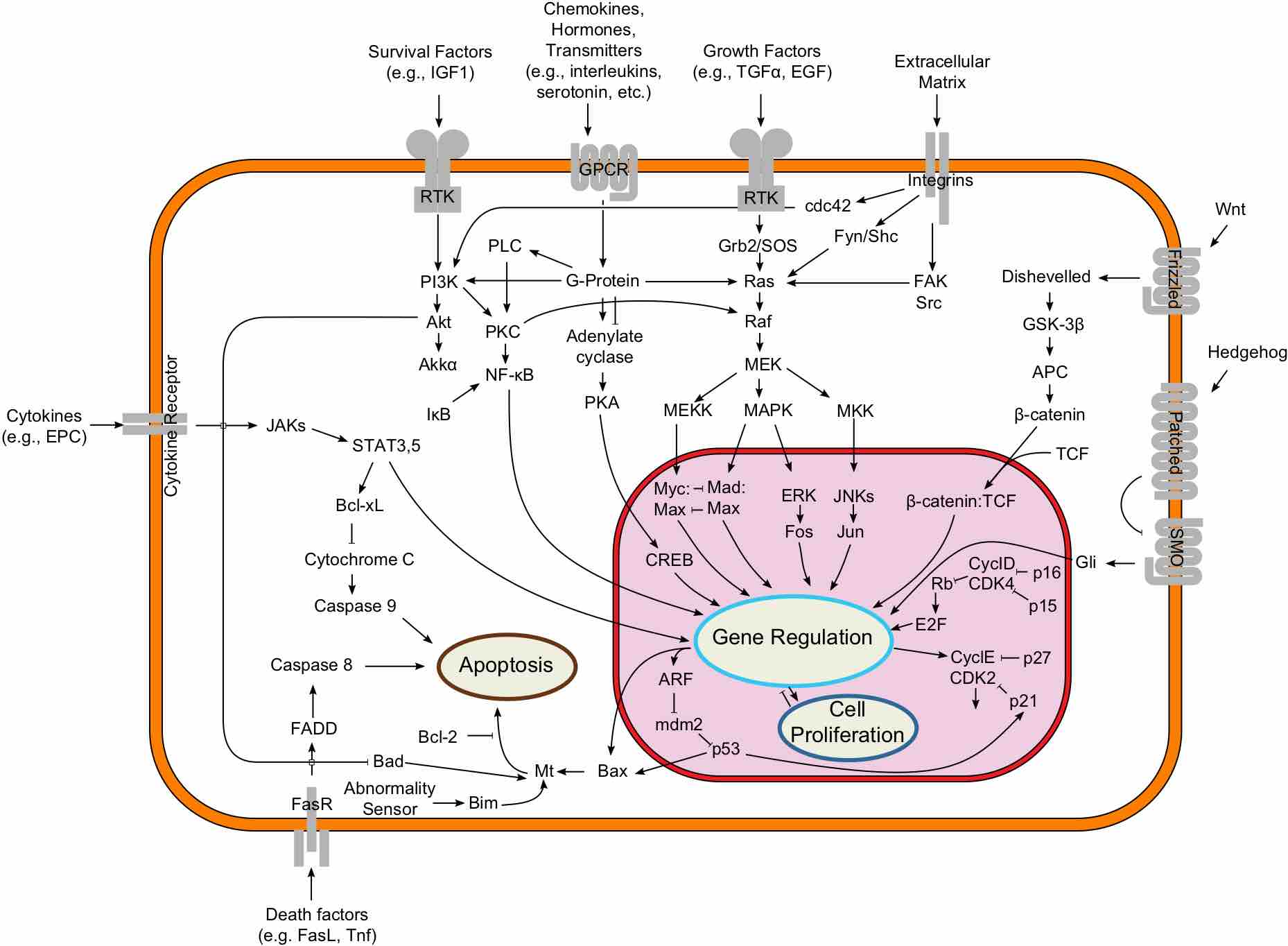
Signal transduction
An overview of major signal transduction pathways.
Two-component signal transduction systems enable bacteria to sense, respond and adapt to a wide range of environments, stressors and growth conditions. Some bacteria can contain as many as 200 two-component systems that need tight regulation to prevent unwanted cross-talk. These pathways have been adapted to respond to a wide variety of stimuli, including nutrients, cellular redox state, changes in osmolarity, quorum signals, antibiotics, temperature, chemoattractants, pH and more. In E. coli the EnvZ/OmpR osmoregulation system controls the differential expression of the outer membrane porin proteins OmpF and OmpC. The KdpD sensor kinase proteins regulate the kdpFABC operon responsible for potassium transport in bacteria including E. coli and Clostridium acetobutylicum. The N-terminal domain of this protein forms part of the cytoplasmic region of the protein, which may be the sensor domain responsible for sensing turgor pressure.
System Variants
A variant of the two-component system is the phospho-relay system. Here a hybrid HK autophosphorylates and then transfers the phosphoryl group to an internal receiver domain, rather than to a separate RR protein. The phosphoryl group is then shuttled to histidine phosphotransferase (HPT) and subsequently to a terminal RR, which can evoke the desired response.
Signal transducing histidine kinases are the key elements in two-component signal transduction systems. Examples of histidine kinases are EnvZ, which plays a central role in osmoregulation, and CheA, which plays a central role in the chemotaxis system. Histidine kinases usually have an N-terminal ligand-binding domain and a C-terminal kinase domain, but other domains may also be present. The kinase domain is responsible for the autophosphorylation of the histidine with ATP, the phosphotransfer from the kinase to an aspartate of the response regulator, and (with bifunctional enzymes) the phosphotransfer from aspartyl phosphate back to ADP or to water. The kinase core has a unique fold, distinct from that of the Ser/Thr/Tyr kinase superfamily.
HKs can be roughly divided into two classes: orthodox and hybrid kinases. Most orthodox HKs, typified by the Escherichia coli EnvZ protein, function as periplasmic membrane receptors and have a signal peptide and transmembrane segment(s) that separate the protein into a periplasmic N-terminal sensing domain and a highly conserved cytoplasmic C-terminal kinase core. Members of this family, however, have an integral membrane sensor domain. Not all orthodox kinases are membrane bound, e.g., the nitrogen regulatory kinase NtrB (GlnL) is a soluble cytoplasmic HK. Hybrid kinases contain multiple phosphodonor and phosphoacceptor sites and use multi-step phospho-relay schemes instead of promoting a single phosphoryl transfer. In addition to the sensor domain and kinase core, they contain a CheY-like receiver domain and a His-containing phosphotransfer (HPt) domain.
The Hpr Serine/threonine kinase PtsK is the sensor in a multicomponent phosphorelay system in control of carbon catabolic repression in bacteria. This kinase is unusual in that it recognizes the tertiary structure of its target and is a member of a novel family unrelated to any previously described protein phosphorylating enzymes. X-ray analysis of the full-length crystalline enzyme from Staphylococcus xylosus at a resolution of 1.95 A shows the enzyme to consist of two clearly separated domains that are assembled in a hexameric structure resembling a three-bladed propeller. The blades are formed by two N-terminal domains each, and the compact central hub assembles the C-terminal kinase domains.