Chapter 25
Body Fluids and Acid-Base Balance
By Boundless
Fluid can enter the body as preformed water, ingested food and drink, and to a lesser extent, as metabolic water.
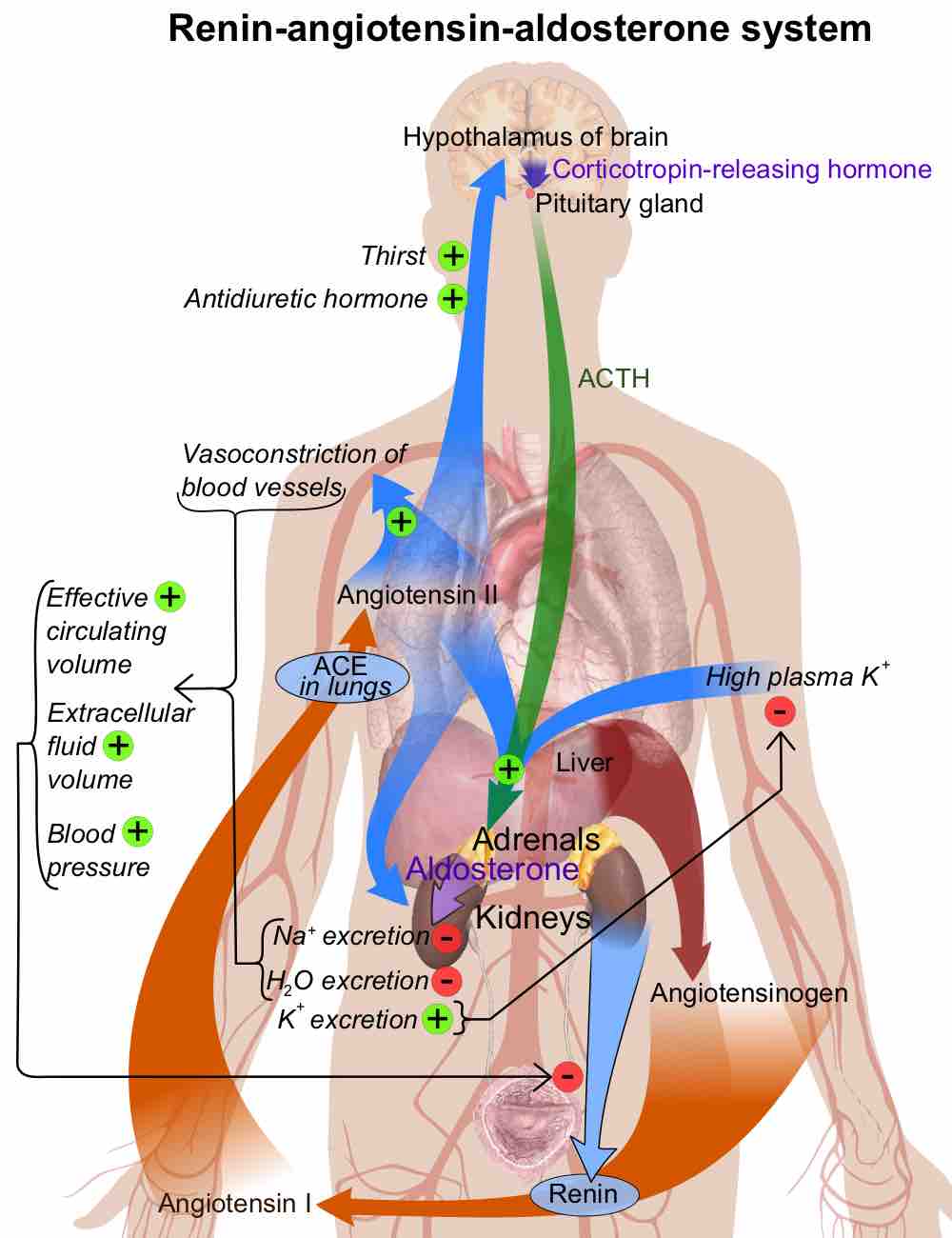
Fluid can leave the body in several ways: urination, exretion (feces), and perspiration (sweating).
Urea, a nitrogenous waste material, is the end product excreted in urine when ammonia is metabolized by animals, such as mammals.
Dehydration is the excessive loss of body fluid.
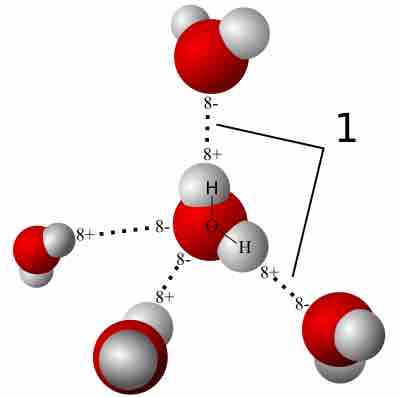
A significant percentage of the human body is water, which includes intracellular and extracellular fluids.
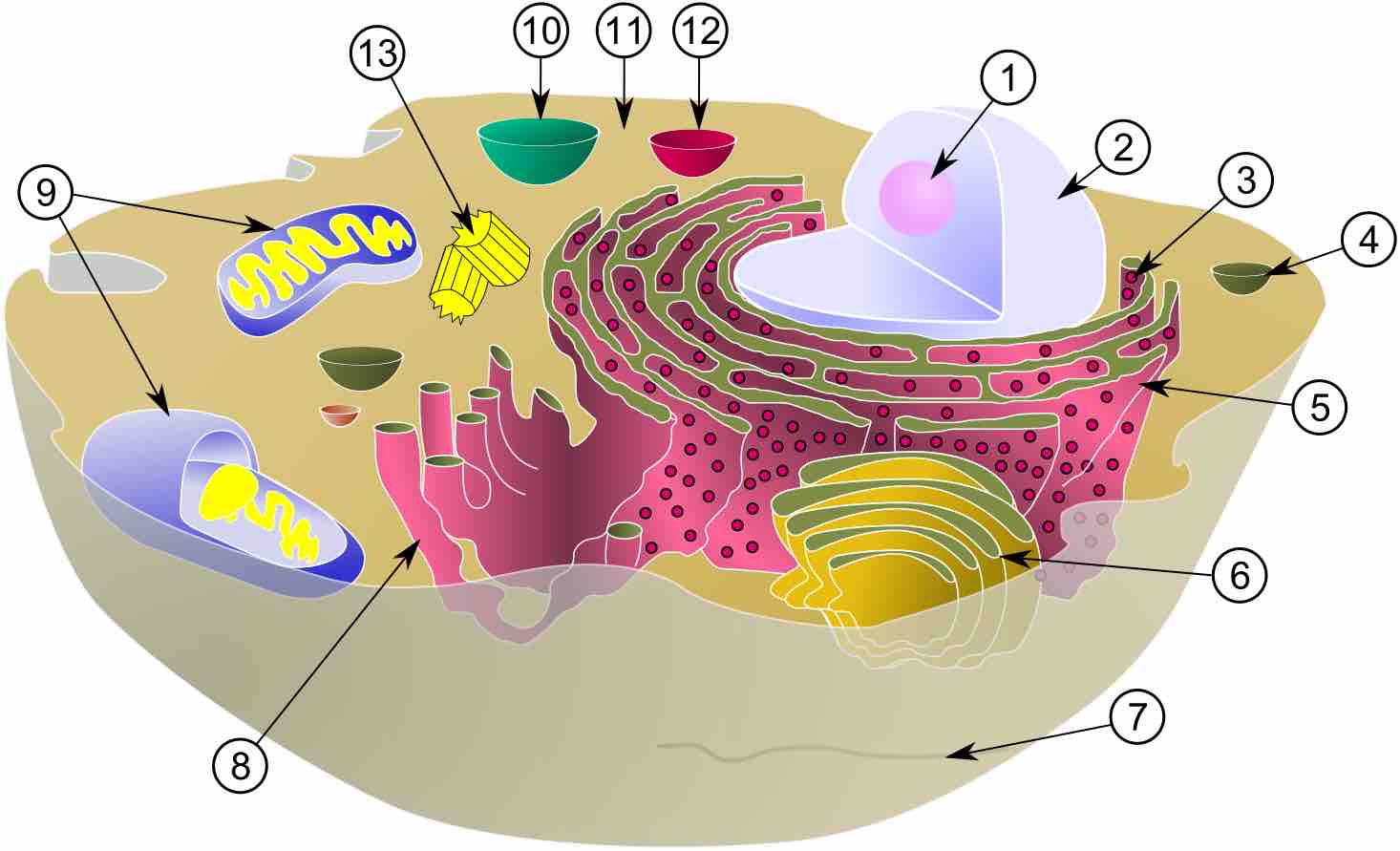
The major body fluid compartments include: intracellular fluid and extracellular fluid (plasma, interstitial fluid, and trancellular fluid).
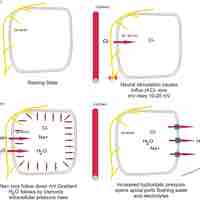
The composition of tissue fluid depends upon the exchanges between the cells in the biological tissue and the blood.
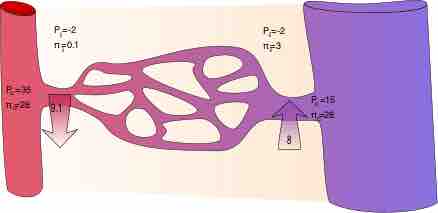
Movement of fluid among compartments depends on several variables described by Starling's equation.
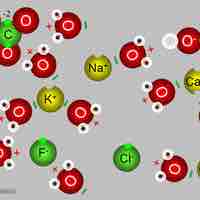
Electrolytes play a vital role in maintaining homeostasis within the body.
Sodium is an important cation distributed primarily outside the cell.
Potassium is mainly an intracellular ion.
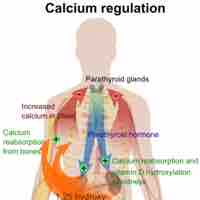
Calcium is a key electrolyte: 99% is deposited in bone and the remainder is associated with hormone release and cell signalling.
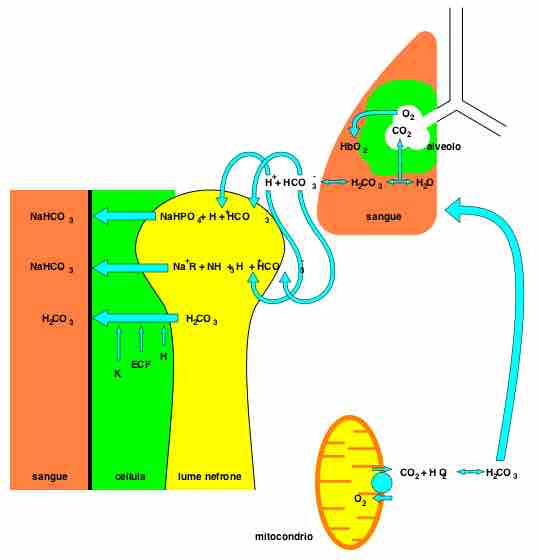
Anions chloride, bicarbonate, and phosphate have important roles in maintaining balances and neutrality of vital mechanisms in the body.
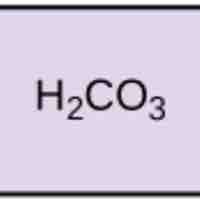
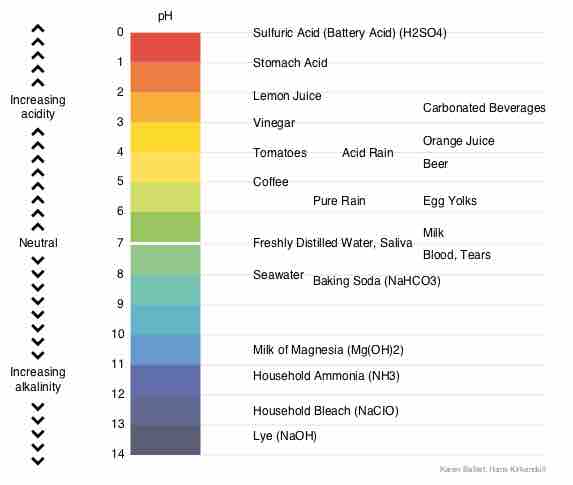
Acids dissociate into H+ and lower pH, while bases dissociate into OH- and raise pH; buffers can absorb these excess ions to maintain pH.
Chemical buffers such as bicarbonate and ammonia help keep blood pH in the narrow range compatible with life.
Acid-base imbalances in blood pH can be altered by changes in breathing to expel more CO2, which will raise pH back to normal.
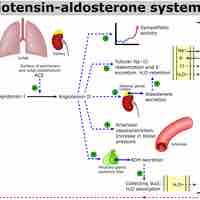
The kidneys help maintain acid-base balance by excreting hydrogen ions into the urine and reabsorbing bicarbonate from the urine.