Importance of Electrolyte Balance
Electrolytes play a vital role in maintaining homeostasis within the body. They help regulate myocardial and neurological function, fluid balance, oxygen delivery, acid-base balance, and other biological processes. Electrolytes are important because they are what cells (especially those of the nerve, heart, and muscle) use to maintain voltages across their cell membranes and to carry electrical impulses (nerve impulses, muscle contractions) across themselves and to other cells .
Electrolyte imbalances can develop from excessive or diminished ingestion and from excessive or diminished elimination of an electrolyte. The most common cause of electrolyte disturbances is renal failure. The most serious electrolyte disturbances involve abnormalities in the levels of sodium, potassium, and/or calcium. Other electrolyte imbalances are less common, and often occur in conjunction with major electrolyte changes. Chronic laxative abuse or severe diarrhea or vomiting (gastroenteritis) can lead to electrolyte disturbances along with dehydration. People suffering from bulimia or anorexia nervosa are especially at high risk for an electrolyte imbalance.
Kidneys work to keep the electrolyte concentrations in blood constant despite changes in your body. For example, during heavy exercise electrolytes are lost in sweat, particularly sodium and potassium, and sweating can increase the need for electrolyte (salt) replacement. It is necessary to replace these electrolytes to keep their concentrations in the body fluids constant.
Dehydration
There are three types of dehydration: hypotonic or hyponatremic (primarily a loss of electrolytes, sodium in particular), hypertonic or hypernatremic (primarily a loss of water), and isotonic or isonatremic (equal loss of water and electrolytes).
In humans, the most common type of dehydration by far is isotonic (isonatraemic) dehydration which effectively equates with hypovolemia; but the distinction of isotonic from hypotonic or hypertonic dehydration may be important when treating people with dehydration. Physiologically, and despite the name, dehydration does not simply mean loss of water, as both water and solutes (mainly sodium) are usually lost in roughly equal quantities as to how they exist in blood plasma. In hypotonic dehydration, intravascular water shifts to the extravascular space, exaggerating intravascular volume depletion for a given amount of total body water loss. Neurological complications can occur in hypotonic and hypertonic states. The former can lead to seizures, while the latter can lead to osmotic cerebral edema upon rapid rehydration.
In more severe cases, correction of a dehydrated state is accomplished by the replenishment of necessary water and electrolytes (through oral rehydration therapy or fluid replacement by intravenous therapy). As oral rehydration is less painful, less invasive, less expensive, and easier to provide, it is the treatment of choice for mild dehydration. Solutions used for intravenous rehydration must be isotonic or hypotonic.
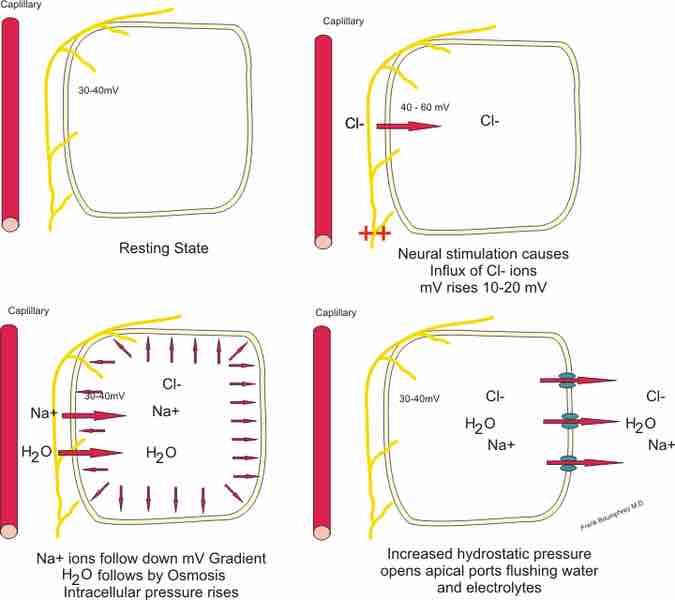
Cell Electrolytes
Illustrating mechanism for transportation of water and electrolytes across epithelial cells in secretory glands.