Amino acids are the structural units that make up proteins. They join together to form short polymer chains called peptides or longer chains called either polypeptides or proteins. These polymers are linear and unbranched, with each amino acid within the chain attached to two neighboring amino acids. The process of making proteins is called translation and involves the step-by-step addition of amino acids to a growing protein chain by a ribozyme that is called a ribosome. The order in which the amino acids are added is read through the genetic code from an mRNA template, which is a RNA copy of one of the organism's genes.
Twenty-two amino acids are naturally incorporated into polypeptides and are called proteinogenic or natural amino acids. Of these, 20 are encoded by the universal genetic code. The remaining two, selenocysteine and pyrrolysine, are incorporated into proteins by unique synthetic mechanisms. Selenocysteine is incorporated when the mRNA being translated includes a SECIS element, which causes the UGA codon to encode selenocysteine instead of a stop codon. Pyrrolysine is used by some methanogenic archaea in enzymes that they use to produce methane. It is coded with the codon UAG, which is normally a stop codon in other organisms. Pyrrolysine (abbreviated as Pyl or O) is a naturally occurring amino acid similar to lysine, but with an added pyrroline ring linked to the end of the lysine side chain . Produced by a specific tRNA and aminoacyl tRNA synthetase, it forms part of an unusual genetic code in these organisms. It is considered the 22nd proteinogenic amino acid. This UAG codon is followed by a PYLIS downstream sequence.
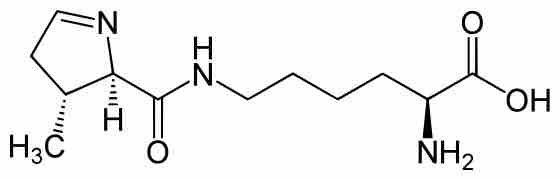
Structure of Pyrrolysine
Pyrrolysine (abbreviated as Pyl or O) is a naturally occurring, genetically coded amino acid used by some methanogenic archaea and one known bacterium in enzymes that are part of their methane-producing metabolism.
Organisms vary in their ability to synthesize the 20 common amino acids. Most bacteria and plants can synthesize all 20. Some simple parasites, such as the bacteria Mycoplasma pneumoniae, lack all amino acid synthesis and take their amino acids directly from their hosts. All amino acids are synthesized from intermediates in glycolysis, the citric acid cycle, or the pentose phosphate pathway. Nitrogen is provided by glutamate and glutamine. Amino acid synthesis depends on the formation of the appropriate alpha-keto acid, which is then transaminated to form an amino acid. Amino acids are made into proteins by being joined together in a chain by peptide bonds. Each different protein has a unique sequence of amino acid residues: this is its primary structure. Just as the letters of the alphabet can be combined to form an almost endless variety of words, amino acids can be linked in varying sequences to form a huge variety of proteins. Proteins are made from amino acids that have been activated by attachment to a transfer RNA molecule through an ester bond.
Aside from the 22 standard amino acids, there are many other amino acids that are called non-proteinogenic or non-standard. Those either are not found in proteins (for example carnitine, GABA) or are not produced directly and in isolation by standard cellular machinery (for example, hydroxyproline and selenomethionine). Non-standard amino acids that are found in proteins are formed by post-translational modification, which is modification after translation during protein synthesis. These modifications are often essential for the function or regulation of a protein. For example, the carboxylation of glutamate allows for better binding of calcium cations. The hydroxylation of proline is critical for maintaining connective tissues. Another example is the formation of hypusine in the translation initiation factor EIF5A, through modification of a lysine residue. Such modifications can also determine the localization of the protein, e.g., the addition of long hydrophobic groups can cause a protein to bind to a phospholipid membrane. Some nonstandard amino acids are not found in proteins. Examples include lanthionine, 2-aminoisobutyric acid, dehydroalanine, and the neurotransmitter gamma-aminobutyric acid. Nonstandard amino acids often occur as intermediates in the metabolic pathways for standard amino acids — for example, ornithine and citrulline occur in the urea cycle, part of amino acid catabolism.