The Complement System
The serum complement system, which represents a chief component of innate immunity, not only participates in inflammation but also acts to enhance the adaptive immune response. Specific activation of the complement via innate recognition proteins or secreted antibody releases cleavage products that interact with a wide range of cell surface receptors found on myeloid, lymphoid, and stromal cells. This intricate interaction among complement activation products and cell surface receptors provides a basis for the regulation of both B and T cell responses.
The complement system plays a crucial role in the innate defense against common pathogens. Activation of the complement leads to robust and efficient proteolytic cascades, which terminate in opsonization and lysis of the pathogen as well as in the generation of the classical inflammatory response through the production of potent proinflammatory molecules. More recently, however, the role of the complement in the immune response has been expanded due to observations that link complement activation to adaptive immune responses. It is now understood that the complement is a functional bridge between innate and adaptive immune responses that allows an integrated host defense to pathogenic challenges.
Activation of the Complement System
The complement system can be activated through three major pathways: classical, lectin, and alternative. Initiation of the classical pathway occurs when C1q, in complex with C1r and C1s serine proteases (the C1 complex), binds to the Fc region of complement-fixing antibodies (generally IgG1and IgM) attached to pathogenic surfaces. Autocatalytic activation of C1r and C1s in turn cleaves C4 and C2 into larger (C4b, C2a) and smaller (C4a, C2b) fragments. The larger fragments associate to form C4bC2a on pathogenic surfaces, and the complex gains the ability to cleave C3 and is termed the C3 convertase.
Generation of the C3 convertase, which cleaves C3 into the anaphylatoxin C3a and the opsonin C3b, is the point at which all complement activation cascades converge. When C3 is cleaved into C3b, it exposes an internal thioester bond that allows stable covalent binding of C3b to hydroxyl groups on proximate carbohydrates and proteins. This activity underpins the entire complement system by effectively "tagging" microorganisms as foreign, leading to further complement activation on and around the opsonized surface and terminating in the production of anaphylatoxins and assembly of membrane attack complexes .
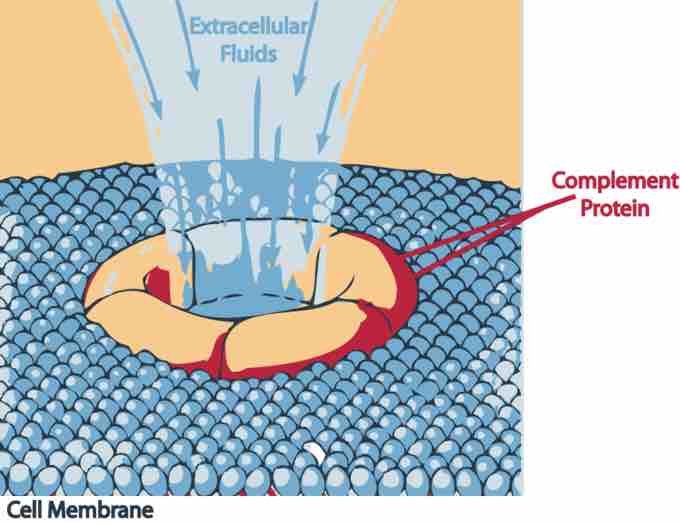
Complement death
A complement protein attacking an invader.
Functions of the Complement System
The functions of the complement system, oposonization, lysis, and generation of the inflammatory response through soluble mediators, are paradigmatic and represent a well-characterized component of an innate host defense. It has become increasingly understood that complement functions in host defense extend beyond innate immune responses. The finding that B lymphocytes bound C3 raised the question as early as in the 1970s as to whether the complement system was involved in adaptive immune responses. Subsequent work demonstrated that depletion of C3 impaired humoral immune responses and provided direct evidence that efficient adaptive responses were contingent on an intact complement system in some cases.
Further study in animals bearing natural complement deficiencies implicated the classical pathway as a crucial mechanism for efficient antigen trapping and retention in lymphoid tissues (e.g., splenic follicles), suggesting that a major function of the complement system was to localize foreign antigens into immune sites important for lymphocytes responses.