Bovine spongiform encephalopathy (BSE), commonly known as mad cow disease, is a fatal neurodegenerative disease in cattle that causes a spongy degeneration in the brain and spinal cord . BSE has a long incubation period, about 30 months to eight years, usually affecting adult cattle at a peak age of four to five years, all breeds being equally susceptible.
Bovine spongiform encephalopathy (BSE)
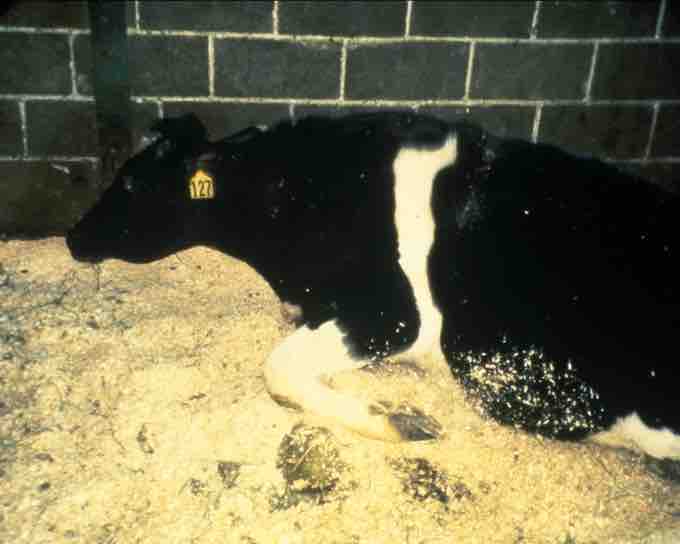
A cow suffering from BSE. The disease has progressed so far the animal cannot stand.
In the United Kingdom, the country worst affected, more than 180,000 cattle have been infected and 4.4 million slaughtered during the eradication program. The disease may be most easily transmitted to human beings by eating food contaminated with the brain, spinal cord or digestive tract of infected carcasses. However, it should also be noted that the infectious agent, although most highly concentrated in nervous tissue, can be found in virtually all tissues throughout the body, including blood. In humans, it is known as new variant Creutzfeldt–Jakob disease (vCJD or nvCJD), and by October 2009, had killed 166 people in the United Kingdom and 44 elsewhere. Between 460,000 and 482,000 BSE-infected animals had entered the human food chain before controls on high-risk offal were introduced in 1989.
The infectious agent in BSE is believed to be a specific type of misfolded protein called a prion. Prions will not disappear even if the beef containing them is cooked. Prion proteins carry the disease between individuals and cause deterioration of the brain.
BSE is a type of transmissible spongiform encephalopathy (TSE). TSEs can arise in animals that carry an allele which causes previously normal protein molecules to contort by themselves from an alpha helix arrangement to a beta sheet, which is the disease-causing shape for the particular protein. Transmission can occur when healthy animals come in contact with tainted tissues from others with the disease. In the brain, these proteins cause native cellular prion protein to deform into the infectious state, which then goes on to deform further prion protein in an exponential cascade. This results in protein aggregates, which then form dense plaque fibers, leading to the microscopic appearance of "holes" in the brain, degeneration of physical and mental abilities, and ultimately death .
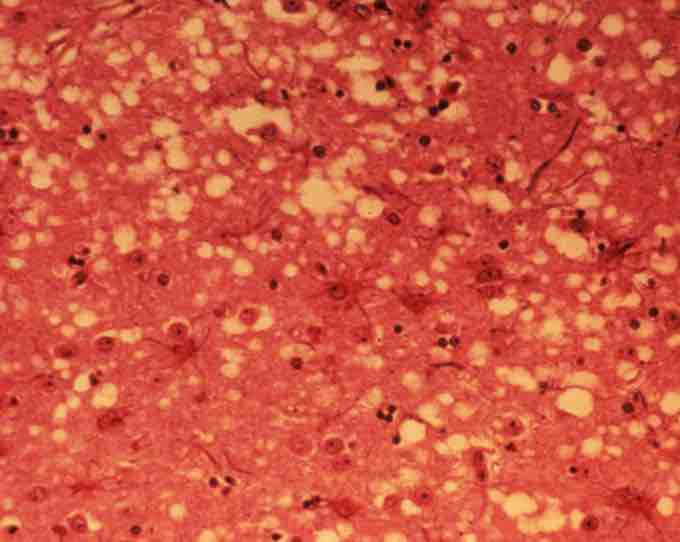
BSE infected brain tissue
This micrograph of brain tissue reveals the cytoarchitectural histopathologic changes found in bovine spongiform encephalopathy. The presence of vacuoles, i.e. microscopic "holes" in the gray matter, gives the brain of BSE-affected cows a sponge-like appearance when tissue sections are examined in the lab.
Different hypotheses exist for the origin of prion proteins in cattle. Two leading hypotheses suggest it may have jumped species from the disease scrapie in sheep, or that it evolved from a spontaneous form of "mad cow disease" that has been seen occasionally in cattle for many centuries. In the fifth century BC, Hippocrates described a similar illness in cattle and sheep, which he believed also occurred in man. Publius Flavius Vegetius Renatus recorded cases of a disease with similar characteristics in the fourth and fifth centuries. Recent research suggests mad cow disease is caused by a genetic mutation within a gene called the prion protein gene. The research shows, for the first time, that a 10-year-old cow from Alabama with an atypical form of bovine spongiform encephalopathy had the same type of prion protein gene mutation as found in human patients with the genetic form of Creutzfeldt–Jakob disease, also called genetic CJD, for short. Besides having a genetic origin, other human forms of prion diseases can be sporadic, as in sporadic CJD, as well as foodborne. They are contracted when people eat products contaminated with mad cow disease. This form of Creutzfeldt-Jakob disease is called variant CJD.