A first line of defense against pathogenic insult is called the innate immune system, which is followed by acquired immune responses associated with the activation of T and B cells aimed against specific antigens. In contrast to the clonal, acquired adaptive immunity, endogenous peptide antibiotics or antimicrobial peptides provide a fast and energy-effective mechanism as front-line defense.
Antimicrobial peptides (AMPs) are small molecular weight proteins with broad spectrum antimicrobial activity against bacteria, viruses, and fungi. They are classified on the basis of their structure and amino acid motifs. Peptides of the defensin, cathelicidin, and histatin classes are found in humans . These evolutionarily conserved peptides are usually positively charged and have both a hydrophobic and hydrophilic side that enables the molecule to be soluble in aqueous environments yet also enter lipid-rich membranes. Once in a target microbial membrane, the peptide kills target cells through diverse mechanisms. AMPs secrete lytic enzymes, nutrient-binding proteins or contain sites that target specific microbial macromolecules.
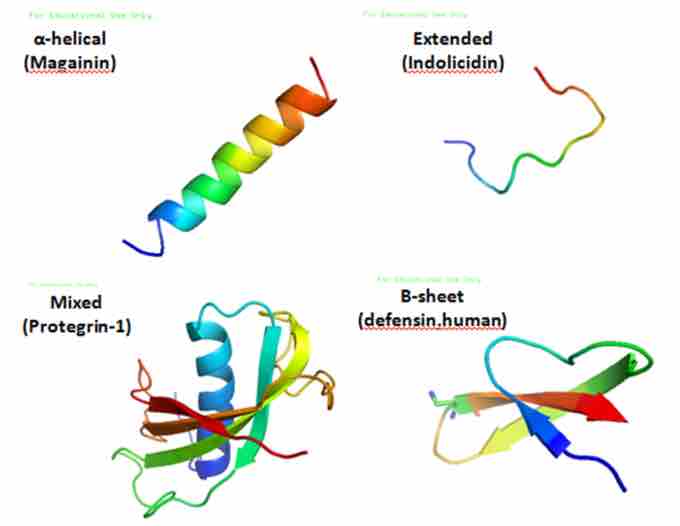
Various AMPs
These are various antimicrobial peptide structures.
Cathelicidins and defensins are major groups of epidermal AMPs. Decreased levels of these peptides have been noted for patients with atopic dermatitis and Kostmann's syndrome, a congenital neutropenia. AMPs have proven effective against multidrug-resistant microbes. In addition to important antimicrobial properties, growing evidence indicates that AMPs alter the host immune response through receptor-dependent interactions. AMPs have been shown to be important in such diverse functions as angiogenesis, wound healing, cytokine release, chemotaxis, and regulation of the adaptive immune system. These peptides qualify as innovative drugs that might be used as antibiotics, anti-lipopolysaccharide drugs, or modifiers of inflammation reactions.