Antisense agents are synthetic, single-stranded short sequences of DNA bases designed to hybridize to specific sequences of messenger RNA (mRNA) forming a duplex . This DNA-RNA coupling attracts an endogenous nuclease, RNase H that destroys the bound RNA and frees the DNA antisense to rehybridize with another copy of mRNA. In this way, the effect is not only highly specific but prolonged because of the recycling of the antisense DNA sequence. When this agent binds to the pathogen DNA or messenger RNA, the biosynthesis of target proteins is disrupted. Therefore, there are at least two ways in which antisense agents act to effectively reduce the amount of pathogenic protein being synthesized - RNase H based degradation of RNA and prevention of ribosomal assembly and translation. This approach has a great advantage. It prevents a pathogenic protein from being produced, rather than trying to selectively neutralize it once it is made.
DNA to Protein
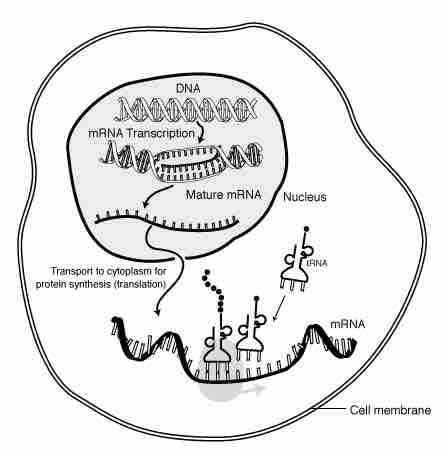
Role of messenger RNA in protein synthesis.
Antisense agents can be specifically targeted to genes that control expression of antibiotic resistance mechanisms, thereby potentially restoring an antibiotic-sensitive phenotype to the cell. A limiting factor in their potential application as therapeutic agents for bacterial infections is their poor uptake by bacterial cells. These agents have been successfully developed for the treatment of viral infections such as cytomegalovirus, hepatitis C, and HIV infections. The advantage of antisense therapy is that they can be manufactured fairly fast, they produce a lasting clinical effect, and they are highly specific to the target. Antisense agents also exhibit efficacy in broader clinical applications such as cancer therapy.