Electrons and Energy
The removal of an electron from a molecule via a process called oxidation results in a decrease in the potential energy stored in the oxidized compound. When oxidation occurs in the cell, the electron (sometimes as part of a hydrogen atom) does not remain un-bonded in the cytoplasm. Instead, the electron shifts to a second compound, reducing the second compound (oxidation of one species always occurs in tandem with reduction of another).
The shift of an electron from one compound to another removes some potential energy from the first compound (the oxidized compound) and increases the potential energy of the second compound (the reduced compound). The transfer of electrons between molecules via oxidation and reduction is important because most of the energy stored in atoms is in the form of high-energy electrons; it is this energy that is used to fuel cellular functions. The transfer of energy in the form of electrons allows the cell to transfer and use energy in an incremental fashion: in small packages rather than as a single, destructive burst.
Electron carriers
In living systems, a small class of molecules functions as electron shuttles: they bind and carry high-energy electrons between compounds in cellular pathways. The principal electron carriers we will consider are derived from the vitamin B group, which are derivatives of nucleotides. These compounds can be easily reduced (that is, they accept electrons) or oxidized (they lose electrons). Nicotinamide adenine dinucleotide (NAD) is derived from vitamin B3, niacin. NAD+ is the oxidized form of niacin; NADH is the reduced form after it has accepted two electrons and a proton (which together are the equivalent of a hydrogen atom with an extra electron). It is noteworthy that NAD+must accept two electrons at once; it cannot serve as a one-electron carrier .
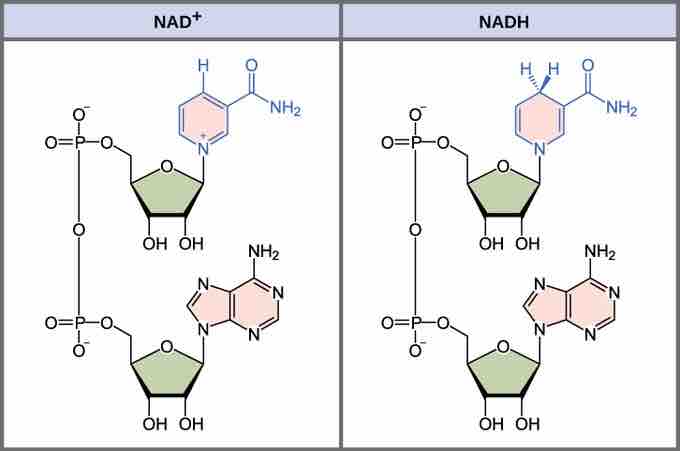
The structure of NADH and NAD+
The oxidized form of the electron carrier (NAD+) is shown on the left and the reduced form (NADH) is shown on the right. The nitrogenous base in NADH has one more hydrogen ion and two more electrons than in NAD+.
NAD+ can accept electrons from an organic molecule according to the general equation:
RH (Reducing agent) + NAD+ (Oxidizing agent) → NADH (Reduced) + R (Oxidized)
When electrons are added to a compound, the compound is reduced. A compound that reduces another is called a reducing agent. In the above equation, RH is a reducing agent and NAD+ is reduced to NADH. When electrons are removed from a compound, the compound is oxidized. In the above equation, NAD+ is an oxidizing agent and RH is oxidized to R. The molecule NADH is critical for cellular respiration and other metabolic pathways.
Similarly, flavin adenine dinucleotide (FAD+) is derived from vitamin B2, also called riboflavin. Its reduced form is FADH2. A second variation of NAD, NADP, contains an extra phosphate group. Both NAD+ and FAD+ are extensively used in energy extraction from sugars, and NADP plays an important role in anabolic reactions and photosynthesis.