ATP in Living Systems
A living cell cannot store significant amounts of free energy. Excess free energy would result in an increase of heat in the cell, which would lead to excessive thermal motion that could damage and then destroy the cell. Rather, a cell must be able to handle that energy in a way that enables the cell to store energy safely and release it for use as needed. Living cells accomplish this by using the compound adenosine triphosphate (ATP) . ATP is often called the "energy currency" of the cell and can be used to fill any energy need of the cell.
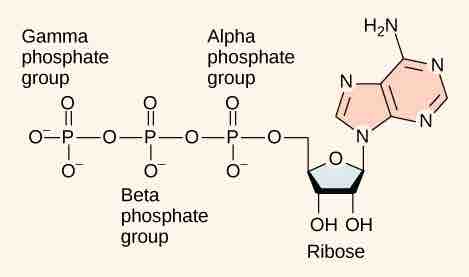
Adenosine triphosphate.
ATP (adenosine triphosphate) has three phosphate groups that can be removed by hydrolysis to form ADP (adenosine diphosphate) or AMP (adenosine monophosphate).The negative charges on the phosphate group naturally repel each other, requiring energy to bond them together and releasing energy when these bonds are broken.
ATP Structure and Function
The core of ATP is a molecule of adenosine monophosphate (AMP), which is composed of an adenine molecule bonded to a ribose molecule and to a single phosphate group. Ribose is a five-carbon sugar found in RNA, and AMP is one of the nucleotides in RNA. The addition of a second phosphate group to this core molecule results in the formation of adenosine diphosphate (ADP); the addition of a third phosphate group forms adenosine triphosphate (ATP).
The addition of a phosphate group to a molecule requires energy. Phosphate groups are negatively charged and, thus, repel one another when they are arranged in a series, as they are in ADP and ATP. This repulsion makes the ADP and ATP molecules inherently unstable. The release of one or two phosphate groups from ATP, a process called dephosphorylation, releases energy.
Energy from ATP
Hydrolysis is the process of breaking complex macromolecules apart. During hydrolysis, water is split, or lysed, and the resulting hydrogen atom (H+) and a hydroxyl group (OH-) are added to the larger molecule. The hydrolysis of ATP produces ADP, together with an inorganic phosphate ion (Pi), and the release of free energy. To carry out life processes, ATP is continuously broken down into ADP, and, like a rechargeable battery, ADP is continuously regenerated into ATP by the reattachment of a third phosphate group. Water, which was broken down into its hydrogen atom and hydroxyl group during ATP hydrolysis, is regenerated when a third phosphate is added to the ADP molecule, reforming ATP.
Obviously, energy must be infused into the system to regenerate ATP. In nearly every living thing on earth, the energy comes from the metabolism of glucose. In this way, ATP is a direct link between the limited set of exergonic pathways of glucose catabolism and the multitude of endergonic pathways that power living cells.
Phosphorylation
When ATP is broken down by the removal of its terminal phosphate group , energy is released and can be used to do work by the cell. Often the released phosphate is directly transferred to another molecule, such as a protein, activating it. For example, ATP supplies the energy to move the contractile muscle proteins during the mechanical work of muscle contraction. Recall the active transport work of the sodium-potassium pump in cell membranes. Phosphorylation by ATP alters the structure of the integral protein that functions as the pump, changing its affinity for sodium and potassium. In this way, the cell performs work, using energy from ATP to pump ions against their electrochemical gradients.
Sometimes phosphorylation of an enzyme leads to its inhibition. For example, the pyruvate dehydrogenase (PDH) complex could be phosphorylated by pyruvate dehydrogenase kinase (PDHK). This reaction leads to inhibition of PDH and its inability to convert pyruvate into acetyl-CoA.
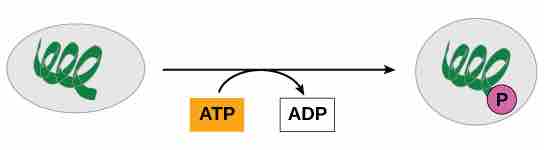
Protein phosphorylation
In phosphorylation reactions, the gamma phosphate of ATP is attached to a protein.
Energy from ATP hydrolysis
The energy from ATP can also be used to drive chemical reactions by coupling ATP hydrolysis with another reaction process in an enzyme. In many cellular chemical reactions, enzymes bind to several substrates or reactants to form a temporary intermediate complex that allow the substrates and reactants to more readily react with each other. In reactions where ATP is involved, ATP is one of the substrates and ADP is a product. During an endergonic chemical reaction, ATP forms an intermediate complex with the substrate and enzyme in the reaction. This intermediate complex allows the ATP to transfer its third phosphate group, with its energy, to the substrate, a process called phosphorylation. Phosphorylation refers to the addition of the phosphate (~P). When the intermediate complex breaks apart, the energy is used to modify the substrate and convert it into a product of the reaction. The ADP molecule and a free phosphate ion are released into the medium and are available for recycling through cell metabolism. This is illustrated by the following generic reaction:
A + enzyme + ATP→[ A enzyme −P ] B + enzyme + ADP + phosphate ion