In our Atom on "Adiabatic Processes" (category: the First Law of Thermodynamics), we learned that an adiabatic process is any process occurring without gain or loss of heat within a system. We also learned a monatomic ideal gas expands adiabatically. In this Atom, we discuss an adiabatic cooling process that can be used to cool a gas, as well as whether absolute zero can be obtained in real systems.
Absolute Zero?
Previously, we learned about the third law of thermodynamics, which states: the entropy of a perfect crystal at absolute zero is exactly equal to zero.
According to the third law, the reason that T=0 cannot be reached is explained as follows: Suppose the temperature of a substance can be reduced in an isentropic process by changing the parameter X from X2 to X1. As an example, one can think of a multistage adiabatic magnetization-demagnetization cycle setup where a magnetic field is switched on and off in a controlled way. (See below. The parameter X in this case would be the magnetization of the gas. )
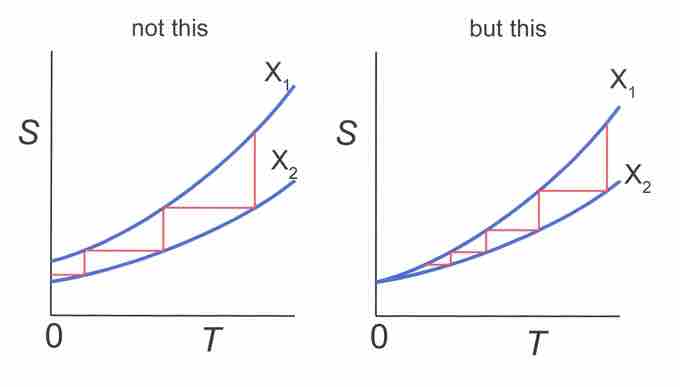
Assuming an entropy difference at absolute zero, T=0 could be reached in a finite number of steps. However, going back to the third law, at T=0 there is no entropy difference, and therefore an infinite number of stepswould be needed for this process (illustrated in ).
Can Absolute Zero be Reached?
Temperature-Entropy diagram. Horizontal lines represent isentropic processes, while vertical lines represent isothermal processes. Left side: Absolute zero can be reached in a finite number of steps if S(T=0,X1)≠S(T=0, X2). Right: An infinite number of steps is needed since S(0,X1)= S(0,X2).
Adiabatic Demagnetization Cooling
In simple terms, the cooling scheme mentioned above occurs by repeating the following steps:
- A strong magnetic field is applied to adiabatically align magnetic moments of the particles in the gas.
- The magnetic field is reduced adiabatically, and thermal energy of the gas causes "ordered" magnetic moments to become random again.
In the second step, thermal energy in the gas is used to cause disorder of magnetic moments. Therefore, the temperature of the gas is reduced (hence cooling works). This scheme is called adiabatic demagnetization cooling.