Concept
Version 8
Created by Boundless
Adiabatic Processes
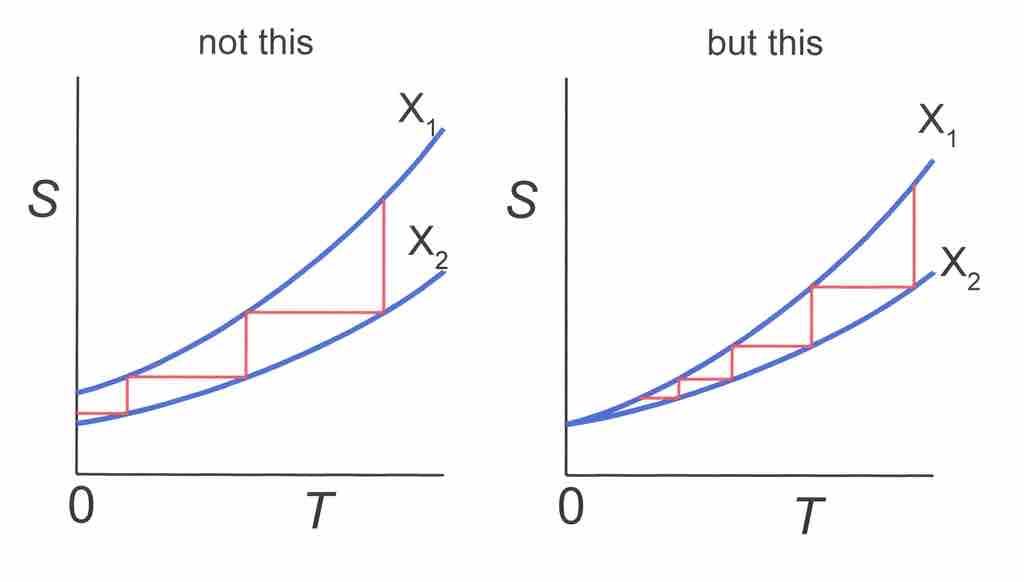
Can Absolute Zero be Reached?
Temperature-Entropy diagram. Horizontal lines represent isentropic processes, while vertical lines represent isothermal processes. Left side: Absolute zero can be reached in a finite number of steps if S(T=0,X1)≠S(T=0, X2). Right: An infinite number of steps is needed since S(0,X1)= S(0,X2).
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"Third law of thermodynamics."
http://en.wikipedia.org/wiki/Third_law_of_thermodynamics
Wikipedia
GNU FDL.