Botulinum toxin is a protein and neurotoxin produced by Clostridium botulinum, C. butyricum, C. baratii and C. argentinense. Botulinum toxin can cause botulism, a serious and life-threatening illness in humans and animals. In 1949, Arnold Burgen's group discovered, through an elegant experiment, that botulinum toxin blocks neuromuscular transmission through decreased acetylcholine release. In 1973, Alan Scott used botulinum toxin type A (BTX-A) in monkey experiments. In 1980, he officially used BTX-A for the first time in humans to treat "crossed eyes" (strabismus), a condition in which the eyes are not properly aligned with each other, as well as "uncontrollable blinking" (blepharospasm). In 1993, Pasricha and colleagues showed that botulinum toxin could be used for the treatment of achalasia, a spasm of the lower esophageal sphincter. In 1994, Bushara showed botulinum toxin injections inhibit sweating; this was the first demonstration of non-muscular use of BTX-A in humans. The cosmetic effect of BTX-A on wrinkles was first reported by J. D. and J. A. Carruthers in a 1992 study on BTX-A for the treatment of glabellar frown lines. The acceptance of BTX-A use for the treatment of muscle pain disorders is growing, with approvals pending in many European countries. The efficacy of BTX-A in treating a variety of other medical conditions (including prostatic dysfunction, asthma, and others) is an area of continued study.
Botulinum Toxin
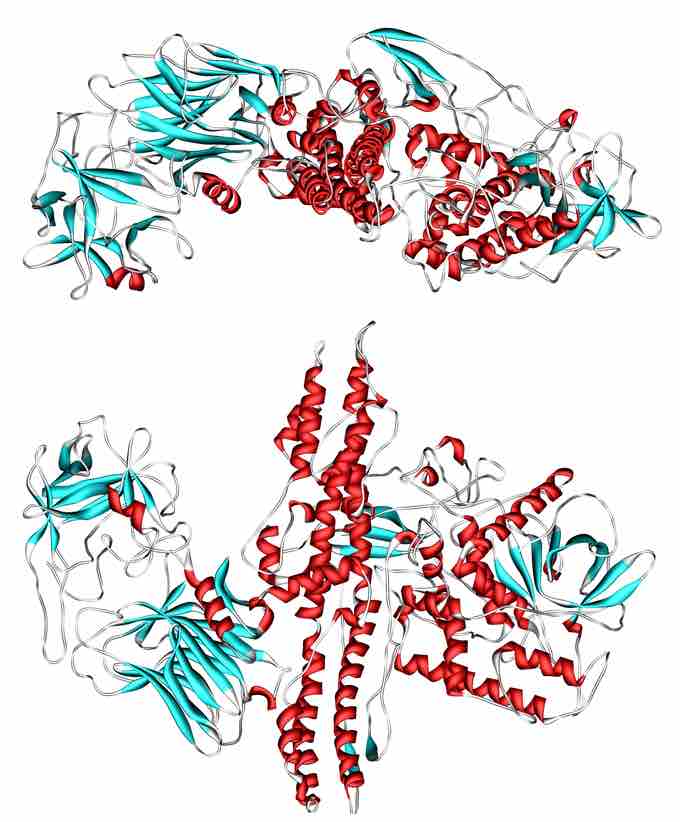
Structure of Botulinum toxin, a protein and neurotoxin produced by the bacterium Clostridium botulinum
Foodborne botulism can be transmitted through food that has not been heated correctly prior to being canned, or food from a can that has not been cooked correctly. Most infant botulism cases cannot be prevented because the bacteria that cause this disease are in soil and dust. The bacteria can also be found inside homes on floors, carpet, and countertops, even after cleaning. Honey can contain the bacteria that cause infant botulism, so children less than 12 months old should not be fed honey.
Botulinum toxin is a two-chain polypeptide with a 100-kDa heavy chain joined by a disulfide bond to a 50-kDa light chain. This light chain is an enzyme (a protease) that attacks one of the fusion proteins (SNAP-25, syntaxin or synaptobrevin) at a neuromuscular junction, preventing vesicles from anchoring to the membrane to release acetylcholine. By inhibiting acetylcholine release, the toxin interferes with nerve impulses and causes flaccid (sagging) paralysis of muscles in botulism, as opposed to the spastic paralysis seen in tetanus. The heavy chain of the toxin is particularly important for targeting the toxin to specific types of axon terminals. The toxin must get inside the axon terminals to cause paralysis. Following the attachment of the toxin heavy chain to proteins on the surface of axon terminals, the toxin can be taken into neurons by endocytosis. The light chain is able to cleave endocytotic vesicles and reach the cytoplasm. The light chain of the toxin has protease activity. The type A toxin proteolytically degrades the SNAP-25 protein, a type of SNARE protein. The SNAP-25 protein is required for vesicle fusion that releases neurotransmitters from the axon endings (in particular acetylcholine). Botulinum toxin specifically cleaves these SNAREs, and so prevents neurosecretory vesicles from docking/fusing with the nerve synapse plasma membrane and releasing their neurotransmitters.