Digestion is a form of catabolism: a breakdown of large food molecules (i.e., polysaccharides, proteins, fats, nucleic acids) to smaller ones (i.e., monosaccharides, amino acids, fatty acids, nucleotides) .
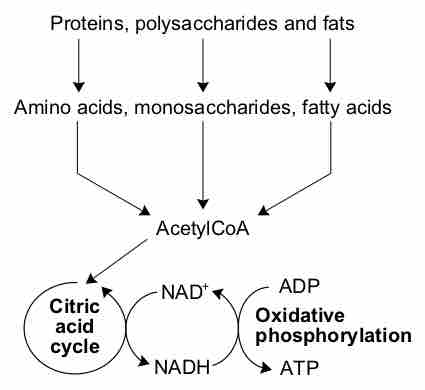
Catabolism
A simplified outline of the catabolism of proteins, carbohydrates and fats
Carbohydrates are taken in mainly in the form of plant carbohydrate (amylose) and animal carbohydrate (glycogen) together with some sugars, mainly disaccharides. In the western diet about 80% is in the form of amylose. Amylose is not highly branched and consists mainly of long chains of glucose linked by α1:4 linkages. Cellulose, the most abundant starch in nature, is formed of β1:4 linkages and cannot be digested in humans, although bacterial action in the colon does breakdown a minute amount. Glycogen is a multi-branched starch with linkages at the 1:4 and 1:6 position. This gives very large granules of multi-branched starch. Both the parotid and pancreatic amylases hydrolyse the 1:4 link, but not the terminal 1:4 links or the 1:6 links. This breaks amylose down into mainly disaccharides, and glycogen with its 1:6 linkages into polysaccharides . The net result of these actions are numerous disaccharides and polysaccharides. Enzymes attached to the enterocycytes of the small intestine break these down to monosaccharides.
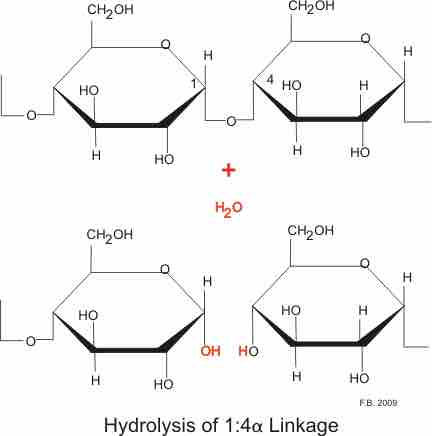
Hydrolysis by amylase
Both the parotid and pancreatic amylases hydrolyse the 1:4 link, but not the terminal 1:4 links or the 1:6 links.
Proteins and polypeptides are digested by hydrolysis of the C-N bond . The proteolytic enzymes are all secreted in an inactive form, to prevent auto-digestion, and are activated in the lumen of the gut: by HCl in the case of the stomach pepsinogen; by enteropeptidase and trypsin in the case of the pancreatic enzymes. Final digestion takes place by small intestine enzymes embedded in the brush border of the small intestine. The enzymes are divided into endo- and exo-peptidases. The endopeptidases cleave the polypeptide at the interior peptide bonds, while the exopeptidases cleave the terminal amino acid. Exopeptidases are further subclassified into aminopeptidases - which cleave off the terminal amino acid at the amine end of the chain, and carboxypeptidases which cleave off the terminal amino acid at the carboxyl end of the chain. Stomach pepsin cleaves interior bonds of the amino acids, and is particularly important for its ability to digest collagen. This is a major constituent of connective tissue of meat. In the absence of stomach pepsin, digestion in the small intestine proceeds with difficulty. Stomach pepsin digests about 20% of the proteins, the rest is digested by pancreatic and small intestine enzymes.
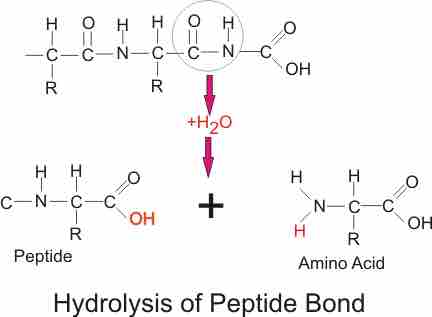
Hydrolysis of peptide bond
Proteins and polypeptides are digested by hydrolysis of the C-N bond.
Fats are digested by lipases which hydrolyze the glycerol-fatty acid bonds. Of particular importance in fat digestion and absorption are bile salts which emulsify the fats allowing for their solution as micelles in chyme, and increasing the surface area on which the pancreatic lipases can operate. Lipases are found in the mouth, the stomach, and the pancreas. Because the lingual lipase was inactivated by stomach acid, it was formally believed to be mainly present for oral hygiene and for its anti-bacterial effect in the mouth. However, it can continue to operate on food stored in the fundus of the stomach, and as much as 30% of the fats can be digested by this lipase. Gastric lipase is of little importance in humans. Pancreatic lipase accounts for the majority of fat digestion and operates in conjuction with the bile salts.
RNA and DNA are hydrolized by pancreatic enzymes (ribonucleases, deoxyribonucleases) into nucleic acids, which are further broken down to purine and pyrimidine bases and pentoses by enzymes in the intestinal mucosa (nucleases).