The complement system helps or "complements" the ability of antibodies and phagocytic cells to clear pathogens from an organism. It is part of the immune system called the "innate immune system" that is not adaptable and does not change over the course of an individual's lifetime. However, it can be recruited and brought into action by the adaptive immune system.
The complement system consists of a number of small proteins found in the blood, generally synthesized by the liver, and normally circulating as inactive precursors (pro-proteins). When stimulated by one of several triggers, proteases in the system cleave specific proteins to release cytokines and initiate an amplifying cascade of further cleavages. The end result of this activation cascade is massive amplification of the response and activation of the cell-killing membrane attack complex. Over 25 proteins and protein fragments make up the complement system, including serum proteins, serosal proteins, and cell membrane receptors. They account for about 5% of the globulin fraction of blood serum.
Three biochemical pathways activate the complement system: the classical complement pathway, the alternative complement pathway, and the lectin pathway. The following are the basic functions of the complement: opsonization (enhancing phagocytosis of antigens); chemotaxis (attracting macrophages and neutrophils); cell lysis (rupturing membranes of foreign cells); and clumping (antigen-bearing agents).
The proteins and glycoproteins that constitute the complement system are synthesized by the liver hepatocytes. But significant amounts are also produced by tissue macrophages, blood monocytes, and epithelial cells of the genitourinal tract and gastrointestinal tract. The three pathways of activation all generate homologous variants of the protease C3-convertase. The classical complement pathway typically requires antigen, antibody complexes for activation (specific immune response), whereas the alternative and mannose-binding lectin pathways can be activated by C3 hydrolysis or antigens without the presence of antibodies (non-specific immune response). In all three pathways, C3-convertase cleaves and activates component C3, creating C3a and C3b, and causing a cascade of further cleavage and activation events. C3b binds to the surface of pathogens, leading to greater internalization by phagocytic cells by opsonization. C5a is an important chemotactic protein, helping recruit inflammatory cells.
C3a is the precursor of an important cytokine (adipokine) named ASP and is usually rapidly cleaved by carboxypeptidase B. Both C3a and C5a have anaphylatoxin activity, directly triggering degranulation of mast cells, as well as increasing vascular permeability and smooth muscle contraction. C5b initiates the membrane attack pathway, which results in the membrane attack complex (MAC), consisting of C5b, C6, C7, C8, and polymeric C9. MAC is the cytolytic endproduct of the complement cascade; it forms a transmembrane channel, which causes osmotic lysis of the target cell. Kupffer cells and other macrophage cell types help clear complement-coated pathogens. As part of the innate immune system, elements of the complement cascade can be found in species earlier than vertebrates, most recently in the protostome horseshoe crab species, putting the origins of the system back further than was previously thought.
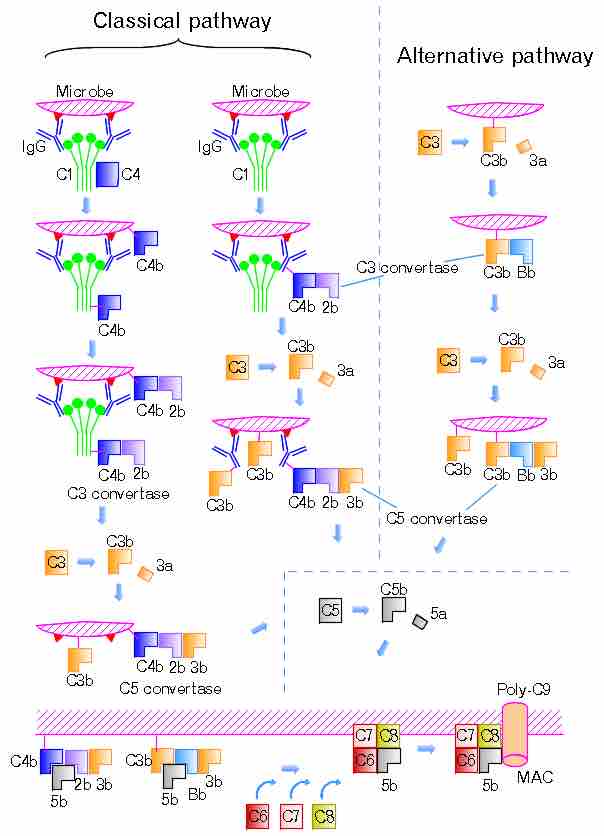
n the classical pathway, C1 binds with its C1q subunits to Fc fragments (made of CH2 region) of IgG or IgM, which forms a complex with antigens. C4b and C3b are also able to bind to antigen-associated IgG or IgM, to its Fc portion.
Such immunoglobulin-mediated binding of the complement may be interpreted, as that the complement uses the ability of the immunoglobulin to detect and bind to non-self antigens as its guiding stick. The complement itself is able to bind non-self pathogens after detecting their pathogen-associated molecular patterns (PAMPs); however, utilizing specificity of antibody, complements are able to detect non-self enemies much more specifically. There must be mechanisms that complements bind to Ig but would not focus its function to Ig but to the antigen.
shows the classical and the alternative pathways with the late steps of complement activation schematically. Some components have a variety of binding sites. In the classical pathway, C4 binds to Ig-associated C1q and C1r2s2 enzyme cleaves C4 to C4b and 4a. C4b binds to C1q, antigen-associated Ig (specifically to its Fc portion), and even to the microbe surface. C3b binds to antigen-associated Ig and to the microbe surface. The ability of C3b to bind to antigen-associated Ig would work effectively against antigen-antibody immune complexes to make them soluble. In the figure, C2b refers to the larger of the C2 fragments.
Complement Pathways
The classical and the alternative pathways with the late steps of complement activation.