The plague is an infectious disease caused by the Gram-negative rod-shaped bacteria Yersinia pestis . Human Y. pestis infection is manifested in three main forms: pneumonic, septicemic, and the notorious bubonic plagues. All three forms are widely believed to have been responsible for a number of high-mortality epidemics throughout human history, including the Plague of Justinian in 542, and the Black Death that accounted for the death of at least one-third of the European population between 1347 and 1353. It has now been conclusively shown that these plagues originated in rodent populations in China. Thousands of cases of the plague are still reported every year; with proper treatment, the prognosis for victims is now much improved. The plague also has a detrimental effect on non-human mammals. In the United States, animals such as the black-tailed prairie dog and the endangered black-footed ferret are under threat from the disease.
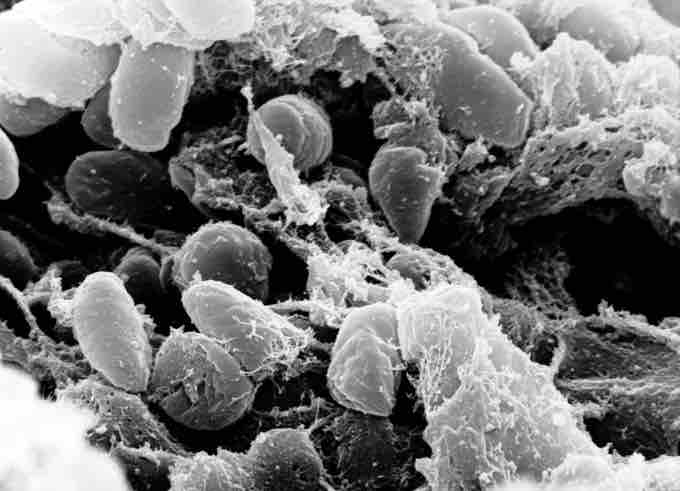
Yersinia pestis
Scanning electron micrograph depicting a mass of Yersinia pestis bacteria (the cause of bubonic plague) in the foregut of the flea vector.
Although bubonic plague is often used synonymously with plague, it refers specifically to an infection that enters through the skin and travels through the lymph nodes (buboes). The incubation period of bubonic plague is from 2-6 days, while the bacteria actively replicate. Symptoms include a lack of energy, fever, headache and chills, and swelling of lymph nodes resulting in buboes, the classic sign of bubonic plague. Septicemic plague is a deadly blood infection; symptoms include hypotension, hepatosplenomegaly, delirium, seizures in children, shock, lethargy, and fever. Pneumonic plague manifests as a severe lung infection, and is more virulent and rare than bubonic plague. Symptoms include fever, chills, coughing, chest pain, dyspnea, hemoptysis, lethargy, hypotension, and shock. Symptoms of the plague are not always present, or the patient may die before any symptoms appear.
Y. pestis is spread most commonly between rodents (both urban and wild) and fleas. Any infected animal can transmit the infection to humans through contact with skin tissue. Humans can also spread the bacteria to other humans through sneezing, coughing, or with direct contact with infected tissue. The reservoir commonly associated with Y. pestis is several species of rodents. In the steppes, the reservoir species is believed to be principally the marmot. In the United States, several species of rodents are thought to maintain Y. pestis. However, the expected disease dynamics have not been found in any rodent species. It is known that rodent populations will have a variable resistance, which could lead to a carrier status in some individuals. In some regions of the world, the reservoir of infection is not clearly identified, which complicates prevention and early warning programs.
The transmission of Y. pestis by fleas is well characterized. Initial acquisition of Y. pestis by the vector occurs during feeding on an infected animal. Several proteins then contribute to the maintenance of the bacteria in the flea digestive tract, among them the hemin storage (Hms) system and Yersinia murine toxin (Ymt). Although Yersinia murine toxin is highly toxic to rodents and was once thought to be produced to ensure reinfection of new hosts, it has been demonstrated that Ymt is important for the survival of Y. pestis in fleas. The Hms system plays an important role in the transmission of Y. pestis back to a mammalian host. While in the insect vector, proteins encoded by Hms genetic loci induce biofilm formation in the proventriculus, a valve connecting the midgut to the esophagus. Aggregation in the biofilm inhibits feeding, as a mass of clotted blood and bacteria forms (referred to as "Bacot's block"). Transmission of Y. pestis occurs during the futile attempts of the flea to feed. Ingested blood is pumped into the esophagus, where it dislodges bacteria lodged in the proventriculus and is regurgitated back into the host circulatory system.

A flea infected with yersinia pestis
A flea infected with yersinia pestis, shown as a dark mass. The foregut of this flea is blocked by a Y. pestis biofilm, which is a prerequisite for efficient transmission.
The pathogenesis of Y. pestis infection in mammalian hosts is due to several factors. The bacteria proliferates inside lymph nodes where it is able to avoid destruction by cells of the immune system such as macrophages. Y. pestis is able to suppress the immune system, avoiding normal immune system responses such as phagocytosis and antibody production. Flea bites allow for the bacteria to pass the skin barrier. Y. pestis expresses the yadBC gene, which is similar to adhesins in other Yersinia species, allowing for adherence and invasion of epithelial cells. Finally, Y. pestis expresses a plasminogen activator that is an important virulence factor for pneumonic plague, which may also degrade on blood clots in order to facilitate systematic invasion. Two important anti-phagocytic antigens, Fraction 1 (F1) and LcrV (V), are both important for virulence. Natural or induced immunity is achieved, therefore, by the production of specific opsonic antibodies against F1 and V antigens.
The traditional first line treatment for Y. pestis has been the antibiotics streptomycin, chloramphenicol, tetracycline, and fluoroquinolones. Antibiotic treatment alone is insufficient for some patients, who may also require circulatory, ventilator, or renal support.