Chemical Modifications, Protein Activity, and Longevity
Proteins can be chemically modified with the addition of methyl, phosphate, acetyl, and ubiquitin groups. The addition or removal of these groups from proteins regulates their activity or the length of time they exist in the cell. Sometimes these modifications can regulate where a protein is found in the cell; for example, in the nucleus, the cytoplasm, or attached to the plasma membrane.
Chemical modifications occur in response to external stimuli such as stress, the lack of nutrients, heat, or ultraviolet light exposure. These changes can alter protein function, epigenetic accessibility, transcription, mRNA stability, or translation; all resulting in changes in expression of various genes. This is an efficient way for the cell to rapidly change the abundance levels of specific proteins in response to the environment. Because proteins are involved in every stage of gene regulation, the phosphorylation of a protein (depending on the protein that is modified) can alter accessibility to the chromosome, can alter translation (by altering transcription factor binding or function), can change nuclear shuttling (by influencing modifications to the nuclear pore complex), can alter RNA stability (by binding or not binding to the RNA to regulate its stability), can modify translation (increase or decrease), or can change post-translational modifications (add or remove phosphates or other chemical modifications). All of these protein activities are affected by the phosphorylation process. The enzymes which are responsible for phosphorylation are known as protein kinases. The addition of a phosphate group to a protein can result in either activation or deactivation; it is protein dependent.
Another example of chemical modifications affecting protein activity include the addition or removal of methyl groups. Methyl groups are added to proteins via the process of methylation; this is the most common form of post-translational modification. The addition of methyl groups to a protein can result in protein-protein interactions that allows for transcriptional regulation, response to stress, protein repair, nuclear transport, and even differentiation processes. Methylation on side chain nitrogens is considered largely irreversible while methylation of the carboxyl groups is potentially reversible. Methylation in the proteins negates the negative charge on it and increases the hydrophobicity of the protein. Methylation on carboxylate side chains covers up a negative charge and adds hydrophobicity. The addition of this chemical group changes the property of the protein and, thus, affects it activity.
The addition of an ubiquitin group to a protein marks that protein for degradation. Ubiquitin acts like a flag indicating that the protein lifespan is complete. These proteins are moved to the proteasome, an organelle that functions to remove proteins to be degraded . One way to control gene expression is to alter the longevity of the protein: ubiquitination shortens a protein's lifespan.
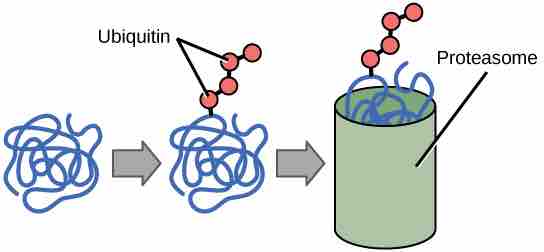
Ubiquitin Tags
Proteins with ubiquitin tags are marked for degradation within the proteasome.