Oxidative phosphorylation is a highly efficient method of producing large amounts of ATP, the basic unit of energy for metabolic processes. During this process electrons are exchanged between molecules, which creates a chemical gradient that allows for the production of ATP. The most vital part of this process is the electron transport chain, which produces more ATP than any other part of cellular respiration.
Electron Transport Chain
The electron transport chain is the final component of aerobic respiration and is the only part of glucose metabolism that uses atmospheric oxygen. Electron transport is a series of redox reactions that resemble a relay race. Electrons are passed rapidly from one component to the next to the endpoint of the chain, where the electrons reduce molecular oxygen, producing water. This requirement for oxygen in the final stages of the chain can be seen in the overall equation for cellular respiration, which requires both glucose and oxygen.
A complex is a structure consisting of a central atom, molecule, or protein weakly connected to surrounding atoms, molecules, or proteins. The electron transport chain is an aggregation of four of these complexes (labeled I through IV), together with associated mobile electron carriers. The electron transport chain is present in multiple copies in the inner mitochondrial membrane of eukaryotes and the plasma membrane of prokaryotes.
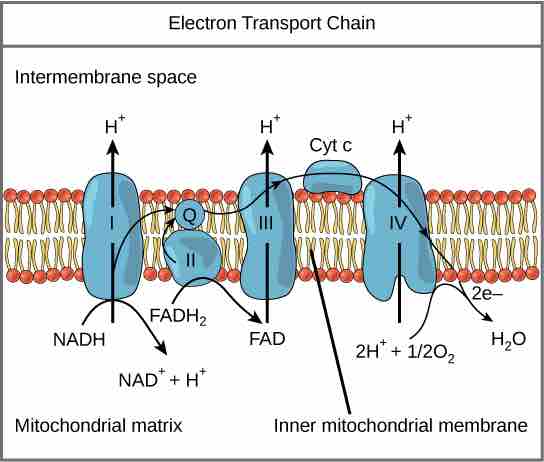
The electron transport chain
The electron transport chain is a series of electron transporters embedded in the inner mitochondrial membrane that shuttles electrons from NADH and FADH2 to molecular oxygen. In the process, protons are pumped from the mitochondrial matrix to the intermembrane space, and oxygen is reduced to form water.
Complex I
To start, two electrons are carried to the first complex aboard NADH. Complex I is composed of flavin mononucleotide (FMN) and an enzyme containing iron-sulfur (Fe-S). FMN, which is derived from vitamin B2 (also called riboflavin), is one of several prosthetic groups or co-factors in the electron transport chain. A prosthetic group is a non-protein molecule required for the activity of a protein. Prosthetic groups can be organic or inorganic and are non-peptide molecules bound to a protein that facilitate its function.
Prosthetic groups include co-enzymes, which are the prosthetic groups of enzymes. The enzyme in complex I is NADH dehydrogenase, a very large protein containing 45 amino acid chains. Complex I can pump four hydrogen ions across the membrane from the matrix into the intermembrane space; it is in this way that the hydrogen ion gradient is established and maintained between the two compartments separated by the inner mitochondrial membrane.
Q and Complex II
Complex II directly receives FADH2, which does not pass through complex I. The compound connecting the first and second complexes to the third is ubiquinone (Q). The Q molecule is lipid soluble and freely moves through the hydrophobic core of the membrane. Once it is reduced to QH2, ubiquinone delivers its electrons to the next complex in the electron transport chain. Q receives the electrons derived from NADH from complex I and the electrons derived from FADH2 from complex II, including succinate dehydrogenase. This enzyme and FADH2 form a small complex that delivers electrons directly to the electron transport chain, bypassing the first complex. Since these electrons bypass, and thus do not energize, the proton pump in the first complex, fewer ATP molecules are made from the FADH2 electrons. The number of ATP molecules ultimately obtained is directly proportional to the number of protons pumped across the inner mitochondrial membrane.
Complex III
The third complex is composed of cytochrome b, another Fe-S protein, Rieske center (2Fe-2S center), and cytochrome c proteins; this complex is also called cytochrome oxidoreductase. Cytochrome proteins have a prosthetic heme group. The heme molecule is similar to the heme in hemoglobin, but it carries electrons, not oxygen. As a result, the iron ion at its core is reduced and oxidized as it passes the electrons, fluctuating between different oxidation states: Fe2+ (reduced) and Fe3+ (oxidized). The heme molecules in the cytochromes have slightly different characteristics due to the effects of the different proteins binding them, which makes each complex. Complex III pumps protons through the membrane and passes its electrons to cytochrome c for transport to the fourth complex of proteins and enzymes. Cytochrome c is the acceptor of electrons from Q; however, whereas Q carries pairs of electrons, cytochrome c can accept only one at a time.
Complex IV
The fourth complex is composed of cytochrome proteins c, a, and a3. This complex contains two heme groups (one in each of the cytochromes a and a3) and three copper ions (a pair of CuA and one CuB in cytochrome a3). The cytochromes hold an oxygen molecule very tightly between the iron and copper ions until the oxygen is completely reduced. The reduced oxygen then picks up two hydrogen ions from the surrounding medium to produce water (H2O). The removal of the hydrogen ions from the system also contributes to the ion gradient used in the process of chemiosmosis.