During chemiosmosis, electron carriers like NADH and FADH donate electrons to the electron transport chain. The electrons cause conformation changes in the shapes of the proteins to pump H+ across a selectively permeable cell membrane. The uneven distribution of H+ ions across the membrane establishes both concentration and electrical gradients (thus, an electrochemical gradient) owing to the hydrogen ions' positive charge and their aggregation on one side of the membrane.
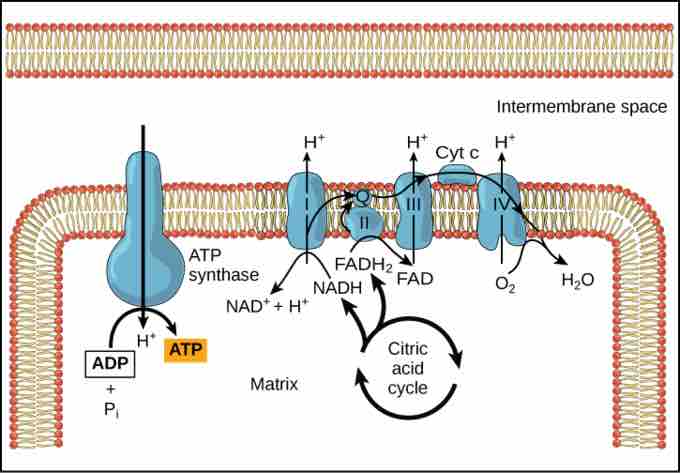
Chemiosmosis
In oxidative phosphorylation, the hydrogen ion gradient formed by the electron transport chain is used by ATP synthase to form ATP.
If the membrane were open to diffusion by the hydrogen ions, the ions would tend to spontaneously diffuse back across into the matrix, driven by their electrochemical gradient. However, many ions cannot diffuse through the nonpolar regions of phospholipid membranes without the aid of ion channels. Similarly, hydrogen ions in the matrix space can only pass through the inner mitochondrial membrane through a membrane protein called ATP synthase. This protein acts as a tiny generator turned by the force of the hydrogen ions diffusing through it, down their electrochemical gradient. The turning of this molecular machine harnesses the potential energy stored in the hydrogen ion gradient to add a phosphate to ADP, forming ATP.
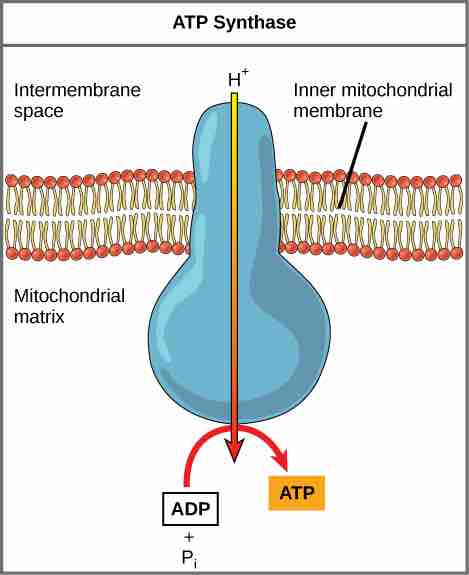
ATP Synthase
ATP synthase is a complex, molecular machine that uses a proton (H+) gradient to form ATP from ADP and inorganic phosphate (Pi).
Chemiosmosis is used to generate 90 percent of the ATP made during aerobic glucose catabolism. The production of ATP using the process of chemiosmosis in mitochondria is called oxidative phosphorylation. It is also the method used in the light reactions of photosynthesis to harness the energy of sunlight in the process of photophosphorylation. The overall result of these reactions is the production of ATP from the energy of the electrons removed from hydrogen atoms. These atoms were originally part of a glucose molecule. At the end of the pathway, the electrons are used to reduce an oxygen molecule to oxygen ions. The extra electrons on the oxygen attract hydrogen ions (protons) from the surrounding medium and water is formed.