Greenhouse gas
Did you know...
The articles in this Schools selection have been arranged by curriculum topic thanks to SOS Children volunteers. To compare sponsorship charities this is the best sponsorship link.
A greenhouse gas (sometimes abbreviated GHG) is a gas in an atmosphere that absorbs and emits radiation within the thermal infrared range. This process is the fundamental cause of the greenhouse effect. The primary greenhouse gases in the Earth's atmosphere are water vapor, carbon dioxide, methane, nitrous oxide, and ozone. In the Solar System, the atmospheres of Venus, Mars, and Titan also contain gases that cause greenhouse effects. Greenhouse gases greatly affect the temperature of the Earth; without them, Earth's surface would average about 33°C colder than the present average of 14 °C (57 °F).
Since the beginning of the Industrial Revolution, the burning of fossil fuels has contributed to a 40% increase in the concentration of carbon dioxide in the atmosphere from 280 ppm to 397 ppm, despite the uptake of a large portion of the emissions by various natural "sinks" involved in the carbon cycle. Anthropogenic carbon dioxide (CO2) emissions (i.e., emissions produced by human activities) come from combustion of carbon based fuels, principally wood, coal, oil, and natural gas.
Gases in Earth's atmosphere
Greenhouse gases
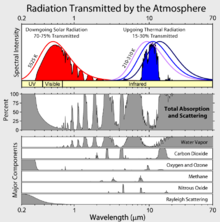
Greenhouse gases are those that can absorb and emit infrared radiation, but not radiation in or near the visible spectrum. In order, the most abundant greenhouse gases in Earth's atmosphere are:
- water vapour (H2O)
- carbon dioxide (CO2)
- methane (CH4)
- nitrous oxide (N2O)
- ozone (O3)
Atmospheric concentrations of greenhouse gases are determined by the balance between sources (emissions of the gas from human activities and natural systems) and sinks (the removal of the gas from the atmosphere by conversion to a different chemical compound). The proportion of an emission remaining in the atmosphere after a specified time is the " Airborne fraction" (AF). More precisely, the annual AF is the ratio of the atmospheric increase in a given year to that year’s total emissions. For CO2 the AF over the last 50 years (1956–2006) has been increasing at 0.25 ± 0.21%/year.
Non-greenhouse gases
Although contributing to many other physical and chemical reactions, the major atmospheric constituents, nitrogen (N2), oxygen (O2), and argon (Ar), are not greenhouse gases. This is because molecules containing two atoms of the same element such as N2 and O2 and monatomic molecules such as Argon (Ar) have no net change in their dipole moment when they vibrate and hence are almost totally unaffected by infrared radiation. Although molecules containing two atoms of different elements such as carbon monoxide (CO) or hydrogen chloride (HCl) absorb IR, these molecules are short-lived in the atmosphere owing to their reactivity and solubility. Because they do not contribute significantly to the greenhouse effect, they are usually omitted when discussing greenhouse gases.
Indirect radiative effects
Some gases have indirect radiative effects (whether or not they are a greenhouse gas themselves). This happens in two main ways. One way is that when they break down in the atmosphere they produce another greenhouse gas. For example methane and carbon monoxide (CO) are oxidised to give carbon dioxide (and methane oxidation also produces water vapour; that will be considered below). Oxidation of CO to CO2 directly produces an unambiguous increase in radiative forcing although the reason is subtle. The peak of the thermal IR emission from the Earth's surface is very close to a strong vibrational absorption band of CO2 (667 cm−1). On the other hand, the single CO vibrational band only absorbs IR at much higher frequencies (2145 cm−1), where the ~300K thermal emission of the surface is at least a factor of ten lower. On the other hand, oxidation of methane to CO2 which requires reactions with the OH radical, produces an instantaneous reduction, since CO2 is a weaker greenhouse gas than methane; but it has a longer lifetime. As described below this is not the whole story, since the oxidations of CO and CH4 are intertwined by both consuming OH radicals. In any case, the calculation of the total radiative effect needs to include both the direct and indirect forcing.
A second type of indirect effect happens when chemical reactions in the atmosphere involving these gases change the concentrations of greenhouse gases. For example, the destruction of non methane volatile organic compounds (NMVOC) in the atmosphere can produce ozone. The size of the indirect effect can depend strongly on where and when the gas is emitted.
Methane has a number of indirect effects in addition to forming CO2. Firstly, the main chemical which destroys methane in the atmosphere is the hydroxyl radical (OH). Methane reacts with OH and so more methane means that the concentration of OH goes down. Effectively, methane increases its own atmospheric lifetime and therefore its overall radiative effect. The second effect is that the oxidation of methane can produce ozone. Thirdly, as well as making CO2 the oxidation of methane produces water; this is a major source of water vapour in the stratosphere which is otherwise very dry. CO and NMVOC also produce CO2 when they are oxidised. They remove OH from the atmosphere and this leads to higher concentrations of methane. The surprising effect of this is that the global warming potential of CO is three times that of CO2. The same process that converts NMVOC to carbon dioxide can also lead to the formation of tropospheric ozone. Halocarbons have an indirect effect because they destroy stratospheric ozone. Finally hydrogen can lead to ozone production and CH4 increases as well as producing water vapour in the stratosphere.
Contribution of clouds to Earth's greenhouse effect
The major non-gas contributor to the Earth's greenhouse effect, clouds, also absorb and emit infrared radiation and thus have an effect on radiative properties of the greenhouse gases. Clouds are water droplets or ice crystals suspended in the atmosphere.
Impacts on the overall greenhouse effect
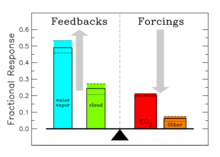
The contribution of each gas to the greenhouse effect is affected by the characteristics of that gas, its abundance, and any indirect effects it may cause. For example, on a kg for kg basis the direct radiative effects of methane is about 72 times stronger than carbon dioxide over a 20 year time frame but it is present in much smaller concentrations so that its total direct radiative effect is smaller, and it has a shorter atmospheric lifetime. On the other hand, in addition to its direct radiative impact methane has a large indirect radiative effect because it contributes to ozone formation. Shindell et al. (2005) argue that the contribution to climate change from methane is at least double previous estimates as a result of this effect.
When ranked by their direct contribution to the greenhouse effect, the most important are:
| Compound |
Formula |
Contribution (%) |
|---|---|---|
| Water vapor and clouds | H2O | 36 – 72% |
| Carbon dioxide | CO2 | 9 – 26% |
| Methane | CH4 | 4 – 9% |
| Ozone | O3 | 3 – 7% |
In addition to the main greenhouse gases listed above, other greenhouse gases include sulfur hexafluoride, hydrofluorocarbons and perfluorocarbons (see IPCC list of greenhouse gases). Some greenhouse gases are not often listed. For example, nitrogen trifluoride has a high global warming potential (GWP) but is only present in very small quantities.
Proportion of direct effects at a given moment
It is not possible to state that a certain gas causes an exact percentage of the greenhouse effect. This is because some of the gases absorb and emit radiation at the same frequencies as others, so that the total greenhouse effect is not simply the sum of the influence of each gas. The higher ends of the ranges quoted are for each gas alone; the lower ends account for overlaps with the other gases. In addition, some gases such as methane are known to have large indirect effects that are still being quantified.
Atmospheric lifetime
Aside from water vapor, which has a residence time of about nine days, major greenhouse gases are well-mixed, and take many years to leave the atmosphere. Although it is not easy to know with precision how long it takes greenhouse gases to leave the atmosphere, there are estimates for the principal greenhouse gases. Jacob (1999) defines the lifetime 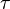 of an atmospheric species X in a one-box model as the average time that a molecule of X remains in the box. Mathematically
of an atmospheric species X in a one-box model as the average time that a molecule of X remains in the box. Mathematically 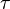 can be defined as the ratio of the mass
can be defined as the ratio of the mass 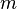 (in kg) of X in the box to its removal rate, which is the sum of the flow of X out of the box (
(in kg) of X in the box to its removal rate, which is the sum of the flow of X out of the box (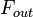 ), chemical loss of X (
), chemical loss of X (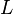 ), and deposition of X (
), and deposition of X (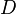 ) (all in kg/sec):
) (all in kg/sec): 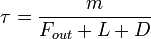 . If one stopped pouring any of this gas into the box, then after a time
. If one stopped pouring any of this gas into the box, then after a time  , its concentration would be about halved.
, its concentration would be about halved.
The atmospheric lifetime of a species therefore measures the time required to restore equilibrium following a sudden increase or decrease in its concentration in the atmosphere. Individual atoms or molecules may be lost or deposited to sinks such as the soil, the oceans and other waters, or vegetation and other biological systems, reducing the excess to background concentrations. The average time taken to achieve this is the mean lifetime.
Carbon dioxide has a variable atmospheric lifetime, and cannot be specified precisely. The atmospheric lifetime of CO2 is estimated of the order of 30–95 years. This figure accounts for CO2 molecules being removed from the atmosphere by mixing into the ocean, photosynthesis, and other processes. However, this excludes the balancing fluxes of CO2 into the atmosphere from the geological reservoirs, which have slower characteristic rates. While more than half of the CO2 emitted is removed from the atmosphere within a century, some fraction (about 20%) of emitted CO2 remains in the atmosphere for many thousands of years. Similar issues apply to other greenhouse gases, many of which have longer mean lifetimes than CO2. E.g., N2O has a mean atmospheric lifetime of 114 years.
Radiative forcing
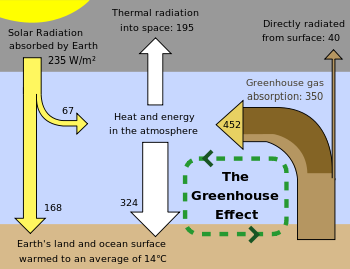
The Earth absorbs some of the radiant energy received from the sun, reflects some of it as light and reflects or re-radiates the rest back to space as heat. The Earth's surface temperature depends on this balance between incoming and outgoing energy. If this energy balance is shifted, the Earth's surface could become warmer or cooler, leading to a variety of changes in global climate.
A number of natural and man-made mechanisms can affect the global energy balance and force changes in the Earth's climate. Greenhouse gases are one such mechanism. Greenhouse gases in the atmosphere absorb and re-emit some of the outgoing energy radiated from the Earth's surface, causing that heat to be retained in the lower atmosphere. As explained above, some greenhouse gases remain in the atmosphere for decades or even centuries, and therefore can affect the Earth's energy balance over a long time period. Factors that influence Earth's energy balance can be quantified in terms of " radiative climate forcing." Positive radiative forcing indicates warming (for example, by increasing incoming energy or decreasing the amount of energy that escapes to space), while negative forcing is associated with cooling.
Global warming potential
The global warming potential (GWP) depends on both the efficiency of the molecule as a greenhouse gas and its atmospheric lifetime. GWP is measured relative to the same mass of CO2 and evaluated for a specific timescale. Thus, if a gas has a high (positive) radiative forcing but also a short lifetime, it will have a large GWP on a 20 year scale but a small one on a 100 year scale. Conversely, if a molecule has a longer atmospheric lifetime than CO2 its GWP will increase with the timescale considered. Carbon dioxide is defined to have a GWP of 1 over all time periods.
Methane has an atmospheric lifetime of 12 ± 3 years and a GWP of 72 over 20 years, 25 over 100 years and 7.6 over 500 years. The decrease in GWP at longer times is because methane is degraded to water and CO2 through chemical reactions in the atmosphere.
Examples of the atmospheric lifetime and GWP relative to CO2 for several greenhouse gases are given in the following table:
| Gas name | Chemical formula |
Lifetime (years) |
Global warming potential (GWP) for given time horizon | ||
|---|---|---|---|---|---|
| 20-yr | 100-yr | 500-yr | |||
| Carbon dioxide | CO2 | See above | 1 | 1 | 1 |
| Methane | CH4 | 12 | 72 | 25 | 7.6 |
| Nitrous oxide | N2O | 114 | 289 | 298 | 153 |
| CFC-12 | CCl2F2 | 100 | 11 000 | 10 900 | 5 200 |
| HCFC-22 | CHClF2 | 12 | 5 160 | 1 810 | 549 |
| Tetrafluoromethane | CF4 | 50 000 | 5 210 | 7 390 | 11 200 |
| Hexafluoroethane | C2F6 | 10 000 | 8 630 | 12 200 | 18 200 |
| Sulfur hexafluoride | SF6 | 3 200 | 16 300 | 22 800 | 32 600 |
| Nitrogen trifluoride | NF3 | 740 | 12 300 | 17 200 | 20 700 |
The use of CFC-12 (except some essential uses) has been phased out due to its ozone depleting properties. The phasing-out of less active HCFC-compounds will be completed in 2030.
Anthropogenic greenhouse gases
Since about 1750 human activity has increased the concentration of carbon dioxide and other greenhouse gases. Measured atmospheric concentrations of carbon dioxide are currently 100 ppm higher than pre-industrial levels. Natural sources of carbon dioxide are more than 20 times greater than sources due to human activity, but over periods longer than a few years natural sources are closely balanced by natural sinks, mainly photosynthesis of carbon compounds by plants and marine plankton. As a result of this balance, the atmospheric mole fraction of carbon dioxide remained between 260 and 280 parts per million for the 10,000 years between the end of the last glacial maximum and the start of the industrial era.
It is likely that anthropogenic (i.e., human-induced) warming, such as that due to elevated greenhouse gas levels, has had a discernible influence on many physical and biological systems. Future warming is projected to have a range of impacts, including sea level rise, increased frequencies and severities of some extreme weather events, loss of biodiversity, and regional changes in agricultural productivity.
The main sources of greenhouse gases due to human activity are:
- burning of fossil fuels and deforestation leading to higher carbon dioxide concentrations in the air. Land use change (mainly deforestation in the tropics) account for up to one third of total anthropogenic CO2 emissions.
- livestock enteric fermentation and manure management, paddy rice farming, land use and wetland changes, pipeline losses, and covered vented landfill emissions leading to higher methane atmospheric concentrations. Many of the newer style fully vented septic systems that enhance and target the fermentation process also are sources of atmospheric methane.
- use of chlorofluorocarbons (CFCs) in refrigeration systems, and use of CFCs and halons in fire suppression systems and manufacturing processes.
- agricultural activities, including the use of fertilizers, that lead to higher nitrous oxide (N2O) concentrations.
The seven sources of CO2 from fossil fuel combustion are (with percentage contributions for 2000–2004):
| Seven main fossil fuel combustion sources |
Contribution (%) |
|---|---|
| Liquid fuels (e.g., gasoline, fuel oil) | 36% |
| Solid fuels (e.g., coal) | 35% |
| Gaseous fuels (e.g., natural gas) | 20% |
| Cement production | 3 % |
| Flaring gas industrially and at wells | < 1% |
| Non-fuel hydrocarbons | < 1% |
| "International bunker fuels" of transport not included in national inventories |
4 % |
Carbon dioxide, methane, nitrous oxide (N2O) and three groups of fluorinated gases ( sulfur hexafluoride (SF6), hydrofluorocarbons (HFCs), and perfluorocarbons (PFCs)) are the major anthropogenic greenhouse gases, and are regulated under the Kyoto Protocol international treaty, which came into force in 2005. Emissions limitations specified in the Kyoto Protocol expire in 2012. The Cancún agreement, agreed in 2010, includes voluntary pledges made by 76 countries to control emissions. At the time of the agreement, these 76 countries were collectively responsible for 85% of annual global emissions.
Although CFCs are greenhouse gases, they are regulated by the Montreal Protocol, which was motivated by CFCs' contribution to ozone depletion rather than by their contribution to global warming. Note that ozone depletion has only a minor role in greenhouse warming though the two processes often are confused in the media.
Role of water vapor
Water vapor accounts for the largest percentage of the greenhouse effect, between 36% and 66% for clear sky conditions and between 66% and 85% when including clouds. Water vapor concentrations fluctuate regionally, but human activity does not significantly affect water vapor concentrations except at local scales, such as near irrigated fields. The atmospheric concentration of vapor is highly variable and depends largely on temperature, from less than 0.01% in extremely cold regions up to 20% in warm, humid regions.
The average residence time of a water molecule in the atmosphere is only about nine days, compared to years or centuries for other greenhouse gases such as CH4 and CO2. Thus, water vapor responds to and amplifies effects of the other greenhouse gases. The Clausius-Clapeyron relation establishes that air can hold more water vapor per unit volume when it warms. This and other basic principles indicate that warming associated with increased concentrations of the other greenhouse gases also will increase the concentration of water vapor (assuming that the relative humidity remains approximately constant; modeling and observational studies find that this is indeed so). Because water vapor is a greenhouse gas, this results in further warming and so is a " positive feedback" that amplifies the original warming. Eventually other earth processes offset these positive feedbacks, stabilizing the global temperature at a new equilibrium and preventing the loss of Earth's water through a Venus-like runaway greenhouse effect.
Again assuming constant relative humidity, the Clausius-Clapeyron equation shows that water vapour increases roughly exponentially with temperature, at approximately 7% for typical temperatures.
Removal from the atmosphere ("sinks")
Natural processes
Greenhouse gases can be removed from the atmosphere by various processes, as a consequence of:
- a physical change (condensation and precipitation remove water vapor from the atmosphere).
- a chemical reaction within the atmosphere. For example, methane is oxidized by reaction with naturally occurring hydroxyl radical, OH· and degraded to CO2 and water vapor (CO2 from the oxidation of methane is not included in the methane Global warming potential). Other chemical reactions include solution and solid phase chemistry occurring in atmospheric aerosols.
- a physical exchange between the atmosphere and the other compartments of the planet. An example is the mixing of atmospheric gases into the oceans.
- a chemical change at the interface between the atmosphere and the other compartments of the planet. This is the case for CO2, which is reduced by photosynthesis of plants, and which, after dissolving in the oceans, reacts to form carbonic acid and bicarbonate and carbonate ions (see ocean acidification).
- a photochemical change. Halocarbons are dissociated by UV light releasing Cl· and F· as free radicals in the stratosphere with harmful effects on ozone (halocarbons are generally too stable to disappear by chemical reaction in the atmosphere).
Negative emissions
A number of technologies remove greenhouse gases emissions from the atmosphere. Most widely analysed are those that remove carbon dioxide from the atmosphere, either to geologic formations such as bio-energy with carbon capture and storage and carbon dioxide air capture, or to the soil as in the case with biochar. The IPCC has pointed out that many long-term climate scenario models require large scale manmade negative emissions to avoid serious climate change.
History of scientific research
Late 19th century scientists experimentally discovered that N2 and O2 do not absorb infrared radiation (called, at that time, "dark radiation") while, on the contrary, water, both as true vapour and condensed in the form of microscopic droplets suspended in clouds, as well as CO2 and other poly-atomic gaseous molecules, do absorb infrared radiation. It was recognized in the early 20th century that greenhouse gases in the atmosphere made the Earth's overall temperature higher than it would be without them. During the late 20th century, a scientific consensus evolved that increasing concentrations of greenhouse gases in the atmosphere are causing a substantial rise in global temperatures and changes to other parts of the climate system, with consequences for the environment and for human health.