Ozone
About this schools Wikipedia selection
SOS Children, which runs nearly 200 sos schools in the developing world, organised this selection. Do you want to know about sponsoring? See www.sponsorachild.org.uk
| Ozone | |
|---|---|
 |
|
 |
|
 |
|
|
Trioxygen |
|
| Identifiers | |
| CAS number | 10028-15-6 |
| Properties | |
| Molecular formula | O3 |
| Molar mass | 47.998 g·mol−1 |
| Appearance | bluish colored gas |
| Density | 2.144 g·L−1 (0 °C), gas |
| Melting point |
80.7 K, −192.5 °C |
| Boiling point |
161.3 K, −111.9 °C |
| Solubility in water | 0.105 g·100mL−1 (0 °C) |
| Thermochemistry | |
| Std enthalpy of formation ΔfH |
+142.3 kJ·mol−1 |
| Standard molar entropy S |
237.7 J·K−1.mol−1 |
| Hazards | |
| EU classification | not listed |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
| Infobox references | |
Ozone (O3) is a triatomic molecule, consisting of three oxygen atoms. It is an allotrope of oxygen that is much less stable than the diatomic O2. Ground-level ozone is an air pollutant with harmful effects on the respiratory systems of animals. Ozone in the upper atmosphere filters potentially damaging ultraviolet light from reaching the Earth's surface. It is present in low concentrations throughout the Earth's atmosphere. It has many industrial and consumer applications. Ozone, the first allotrope of a chemical element to be recognized by science, was proposed as a distinct chemical compound by Christian Friedrich Schönbein in 1840, who named it after the Greek word for smell (ozein), from the peculiar odour in lightning storms. The formula for ozone, O3, was not determined until 1865 by Jacques-Louis Soret and confirmed by Schönbein in 1867. The odour from a lightning strike is from ions produced during the rapid chemical changes, not from the ozone itself.
Physical properties
Ozone is a pale blue, poisonous gas with a sharp, cold, irritating odour. Most people can detect about 0.01 ppm in air. Exposure to 0.1 to 1 ppm produces headaches, burning eyes, and irritation to the respiratory passages.
At -112 °C, it forms a dark blue liquid. At temperatures below -193 °C, it forms a violet-black solid.
Structure
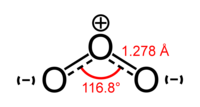
The structure of ozone, according to experimental evidence from microwave spectroscopy, is bent, with C2v symmetry (similar to the water molecule), O – O distance of 127.2 pm and O – O – O angle of 116.78°. The central atom forms an sp² hybridization with one lone pair. Ozone is a polar molecule with a dipole moment of 0.5337 D. The bonding is single bond on one side and double bond on the other side, and these bonds are blended to become known as resonance structures. The bond order is 1.5 for each side.
Chemistry
Ozone is a powerful oxidizing agent, far better than dioxygen. It is also unstable at high concentrations, decaying to ordinary diatomic oxygen (in about half an hour in atmospheric conditions):
- 2 O3 → 3 O2.
This reaction proceeds more rapidly with increasing temperature and decreasing pressure. Deflagration of ozone can be triggered by a spark, and can occur in ozone concentrations of 10 wt% or higher. Ozone will oxidize metals (except gold, platinum, and iridium) to oxides of the metals in their highest oxidation state:
- 2 Cu2+(aq) + 2 H3O+(aq) + O3(g) → 2 Cu3+(aq) + 3 H2O(l) + O2(g)
Ozone also increases the oxidation number of oxides:
- NO + O3 → NO2 + O2
The above reaction is accompanied by chemiluminescence. The NO2 can be further oxidized:
- NO2 + O3 → NO3 + O2
The NO3 formed can react with NO2 to form N2O5:
- NO2 + NO3 → N2O5
Ozone reacts with carbon to form carbon dioxide, even at room temperature:
- C + 2 O3 → CO2 + 2 O2
Ozone does not react with ammonium salts but it reacts with ammonia to form ammonium nitrate:
- 2 NH3 + 4 O3 → NH4NO3 + 4 O2 + H2O
Ozone reacts with sulfides to make sulfates:
- PbS + 4 O3 → PbSO4 + 4 O2
Sulfuric acid can be produced from ozone, starting either from elemental sulfur or from sulfur dioxide:
- S + H2O + O3 → H2SO4
- 3 SO2 + 3 H2O + O3 → 3 H2SO4
All three atoms of ozone may also react, as in the reaction with tin(II) chloride and hydrochloric acid and NaCl along with Ammonium Nitrate:
- 3 SnCl2 + 6 HCl + O3 → 3 SnCl4 + 3 H2O
In the gas phase, ozone reacts with hydrogen sulfide to form sulfur dioxide:
- H2S + O3 → SO2 + H2O
In an aqueous solution, however, two competing simultaneous reactions occur, one to produce elemental sulfur, and one to produce sulfuric acid:
- H2S + O3 → S + O2 + H2O
- 3 H2S + 4 O3 → 3 H2SO4
Iodine perchlorate can be made by treating iodine dissolved in cold anhydrous perchloric acid with ozone:
- I2 + 6 HClO4 + O3 → 2 I(ClO4)3 + 3 H2O
Solid nitryl perchlorate can be made from NO2, ClO2, and O3 gases:
- 2 NO2 + 2 ClO2 + 2 O3 → 2 NO2ClO4 + O2
Ozone can be used for combustion reactions and combusting gases in ozone provides higher temperatures than combusting in dioxygen (O2). Following is a reaction for the combustion of carbon subnitride which can also cause lower temperatures:
- 3 C4N2 + 4 O3 → 12 CO + 3 N2
Ozone can react at cryogenic temperatures. At 77 K (-196 °C), atomic hydrogen reacts with liquid ozone to form a hydrogen superoxide radical, which dimerizes:
- H + O3 → HO2 + O
- 2 HO2 → H2O4
Ozonides can be formed, which contain the ozonide anion, O3-. These compounds are explosive and must be stored at cryogenic temperatures. Ozonides for all the alkali metals are known. KO3, RbO3, and CsO3 can be prepared from their respective superoxides:
- KO2 + O3 → KO3 + O2
Although KO3 can be formed as above, it can also be formed from potassium hydroxide and ozone:
- 2 KOH + 5 O3 → 2 KO3 + 5 O2 + H2O
NaO3 and LiO3 must be prepared by action of CsO3 in liquid NH3 on an ion exchange resin containing Na+ or Li+ ions:
- CsO3 + Na+ → Cs+ + NaO3
Treatment with ozone of calcium dissolved in ammonia leads to ammonium ozonide and not calcium ozonide:
- 3 Ca + 10 NH3 + 6 O3 → Ca•6NH3 + Ca(OH)2 + Ca(NO3)2 + 2 NH4O3 + 2 O2 + H2
Ozone can be used to remove manganese from the water, forming a precipitate which can be filtered:
- 2 Mn2+ + 2 O3 + 4 H2O → 2 MnO(OH)2 (s) + 2 O2 + 4 H+
Ozone will also turn cyanides to the one thousand times less toxic cyanates:
- CN- + O3 → CNO- + O2
Finally, ozone will also completely decompose urea:
- (NH2)2CO + O3 → N2 + CO2 + 2 H2O
Ozone in Earth's atmosphere
The standard way to express total ozone levels (the volume of ozone in a vertical column) in the atmosphere is by using Dobson units. Concentrations at a point are measured in parts per billion (ppb) or in μg/m³.
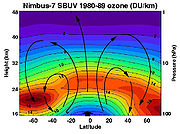
Ozone layer
The highest levels of ozone in the atmosphere are in the stratosphere, in a region also known as the ozone layer between about 10 km and 50 km above the surface (or between 6.21 and 31.1 miles). Here it filters out photons with shorter wavelengths (less than 320 nm) of ultraviolet light, also called UV rays, (270 to 400 nm) from the Sun that would be harmful to most forms of life in large doses. These same wavelengths are also among those responsible for the production of vitamin D, which is essential for human health. Ozone in the stratosphere is mostly produced from ultraviolet rays reacting with oxygen:
- O2 + photon(radiation< 240 nm) → 2 O
- O + O2 → O3
It is destroyed by the reaction with atomic oxygen:
- O3 + O → 2 O2
(See Ozone-oxygen cycle for more detail.)
The latter reaction is catalysed by the presence of certain free radicals, of which the most important are hydroxyl (OH), nitric oxide (NO) and atomic chlorine (Cl) and bromine (Br). In recent decades the amount of ozone in the stratosphere has been declining mostly due to emissions of CFCs and similar chlorinated and brominated organic molecules, which have increased the concentration of ozone-depleting catalysts above the natural background. Ozone only makes up 0.00006% of the atmosphere. See ozone depletion for more information.
Low level ozone
Low level ozone (or tropospheric ozone) is regarded as a pollutant by the World Health Organization. It is not emitted directly by car engines or by industrial operations. It is formed by the reaction of sunlight on air containing hydrocarbons and nitrogen oxides that react to form ozone directly at the source of the pollution or many kilometers down wind. For more details of the complex chemical reactions that produce low level ozone see tropospheric ozone.
Ozone reacts directly with some hydrocarbons such as aldehydes and thus begins their removal from the air, but the products are themselves key components of smog. Ozone photolysis by UV light leads to production of the hydroxyl radical OH and this plays a part in the removal of hydrocarbons from the air, but is also the first step in the creation of components of smog such as peroxyacyl nitrates which can be powerful eye irritants. The atmospheric lifetime of tropospheric ozone is about 22 days and its main removal mechanisms are being deposited to the ground, the above mentioned reaction giving OH, and by reactions with OH and the peroxy radical HO2· (Stevenson et al, 2006).
As well as having an impact on human health (see below) there is also evidence of significant reduction in agricultural yields due to increased ground-level ozone and pollution which interferes with photosynthesis and stunts overall growth of some plant species.
Certain examples of cities with elevated ozone readings are Houston and Mexico City. Houston has a reading of around 41 ppb, while Mexico City is far more hazardous, with a reading of about 125 ppb.
Ozone as a greenhouse gas
Although ozone was present at ground level before the industrial revolution, peak concentrations were far higher than the pre-industrial levels and even background concentrations well away from sources of pollution are substantially higher. This increase in ozone is of further concern as ozone present in the upper troposphere acts as a greenhouse gas, absorbing some of the infrared energy emitted by the earth. Quantifying the greenhouse gas potency of ozone is difficult as it is not present in uniform concentrations across the globe. However, the most recent scientific review on the climate change (the IPCC Third Assessment Report) suggests that the radiative forcing of tropospheric ozone is about 25% that of carbon dioxide.
Ozone cracking
Ozone gas attacks any polymer possessing olefinic or double bonds within its chain structure, such materials including natural rubber, nitrile rubber, and Styrene-butadiene rubber. Products made using these polymers are especially susceptible to the problem, which causes deep and dangerous cracks to grow slowly with time, the rate of crack growth depending on the load carried by the product and the concentration of ozone in the atmosphere. Such products can be protected by adding anti-ozonants, such as waxes which bloom to the surface and create a protective film. Ozone cracks used to be a serious problem in car tires for example, but the problem is now seen only in very old tires.
Ozone and health
Ozone in air pollution
There is a great deal of evidence to show that high concentrations (ppm) of ozone, created by high concentrations of pollution and daylight UV rays at the earth's surface, can harm lung function and irritate the respiratory system. A connection has also been shown to exist between increased ozone caused by thunderstorms and hospital admissions of asthma sufferers. Air quality guidelines such as those from the World Health Organization are based on detailed studies of what levels can cause measurable health effects.
A common British folk myth dating back to the Victorian era holds that the smell of the sea is caused by ozone, and that this smell has "bracing" health benefits. Neither of these is true. The characteristic "smell of the sea" is not caused by ozone, but by the presence of dimethyl sulfide generated by phytoplankton, and dimethyl sulfide, like ozone, is toxic in high concentrations.
The United States Environmental Protection Agency has developed an Air Quality index to help explain air pollution levels to the general public. 8-hour average ozone concentrations of 85 to 104 ppbv are described as "Unhealthy for Sensitive Groups", 105 ppbv to 124 ppbv as "unhealthy" and 125 ppb to 404 ppb as "very unhealthy". The EPA has designated over 300 counties of the United States, clustered around the most heavily populated areas (especially in California and the Northeast), as failing to comply with the National Ambient Air Quality Standards.
Ozone can also be present in indoor air pollution.
Physiology of ozone
Ozone, along with reactive forms of oxygen such as superoxide, singlet oxygen (see oxygen), hydrogen peroxide, and hypochlorite ions, is naturally produced by white blood cells and other biological systems (such as the roots of marigolds) as a means of destroying foreign bodies. Ozone reacts directly with organic double bonds. Also, when ozone breaks down to dioxygen it gives rise to oxygen free radicals, which are highly reactive and capable of damaging many organic molecules. Ozone has been found to convert cholesterol in the blood stream to plaque (which causes hardening and narrowing of arteries). Moreover, it is believed that the powerful oxidizing properties of ozone may be a contributing factor of inflammation. The cause-and-effect relationship of how the ozone is created in the body and what it does is still under consideration and still subject to various interpretations, since other body chemical processes can trigger some of the same reactions. A team headed by Dr. Paul Wentworth Jr. of the Department of Chemistry at the Scripps Research Institute has shown evidence linking the antibody-catalyzed water-oxidation pathway of the human immune response to the production of ozone. In this system, ozone is produced by antibody-catalyzed production of trioxidane from water and neutrophil-produced singlet oxygen. See also trioxidane for more on this biological ozone-producing reaction.
Ozone has also been proven to form specific, cholesterol-derived metabolites that are thought to facilitate the build-up and pathogenesis of atherosclerotic plaques (a form of heart disease). These metabolites have been confirmed as naturally occurring in human atherosclerotic arteries and are categorized into a class of secosterols termed “Atheronals”, generated by ozonolysis of cholesterol's double bond to form a 5,6 secosterol as well as a secondary condensation product via aldolization.
Ozone has been implicated to have an adverse effect on plant growth, "...Ozone reduced total chlorophylls, carotenoid and carbohydrate concentration, and increased 1-aminocyclopropane-1-carboxylic acid (ACC) content and ethylene production. In treated plants, the ascorbate leaf pool was decreased, while lipid peroxidation and solute leakage were significantly higher than in ozone-free controls. The data indicated that ozone triggered protective mechanisms against oxidative stress in citrus."
Use of ozone in medical therapy
The recently-discovered physiologic role of ozone has led ozone medical advocates to suggest this as a mechanism for the use of artificially produced ozone to kill microbes in blood. Inhaled ozone is toxic to lung tissue in small amounts, but ozone appears to be less toxic when directly mixed with blood, either inside or outside the body. However, the utility of ozone in medical therapy (if any) is a subject of debate. No form of ozone therapy is presently approved by the FDA in the U.S.
Preparation
Ozone often forms in nature under conditions where O2 will not react. Ozone used in industry is measured in g/Nm³ or weight percent. The regime of applied concentrations ranges from 1 to 5 weight percent in air and from 6 to 13 weight percent in oxygen.
Corona discharge method
This is the most popular type of ozone generator for most industrial and personal uses. While variations of the "hot spark" coronal discharge method of ozone production exist, including medical grade and industrial grade ozone generators, these units usually work by means of a corona discharge tube. They are typically very cost-effective, and do not require an oxygen source other than the ambient air. However, they also produce nitrogen oxides as a by-product. Use of an air dryer can reduce or eliminate nitric acid formation by removing water vapor and increase ozone production. Use of an oxygen concentrator can further increase the ozone production and further reduce the risk of nitric acid formation due to removing not only the water vapor, but also the bulk of the nitrogen.
Ultraviolet light
UV ozone generators employ a light source that generates the same narrow-band ultraviolet light that is responsible for the sustenance of the ozone layer in the stratosphere of the Earth. While standard UV ozone generators tend to be less expensive, they usually produce ozone with a concentration of about 2% or lower. Another disadvantage of this method is that it requires the air to be exposed to the UV source for a longer amount of time, and any air that is not exposed to the UV source will not be treated. This makes UV generators impractical for use in situations that deal with rapidly moving air or water streams (in-duct air sterilization, for example).
Cold plasma
In the cold plasma method, pure oxygen gas is exposed to a plasma created by dielectric barrier discharge. The diatomic oxygen is split into single atoms, which then recombine in triplets to form ozone.
Cold plasma machines utilize pure oxygen as the input source, and produce a maximum concentration of about 5% ozone. They produce far greater quantities of ozone in a given space of time compared to ultraviolet production. However, because cold plasma ozone generators are very expensive, and still require occasional maintenance, they are found less frequently than the previous two types.
The discharges manifest as filamentary transfer of electrons (micro discharges) in a gap between two electrodes. In order to evenly distribute the micro discharges, a dielectric insulator must be used to separate the metallic electrodes and to prevent arcing.
Some cold plasma units also have the capability of producing short-lived allotropes of oxygen which include O4, O5, O6, O7, etc. These anions are even more reactive than ordinary O3.
Special considerations
Ozone cannot be stored and transported like other industrial gases (because it quickly decays into diatomic oxygen) and must therefore be produced on site. Available ozone generators vary in the arrangement and design of the high-voltage electrodes. At production capacities higher than 20 kg per hour, a gas/water tube heat-exchanger is utilized as ground electrode and assembled with tubular high-voltage electrodes on the gas-side. The regime of typical gas pressures is around 2 bar absolute in oxygen and 3 bar absolute in air. Several megawatts of electrical power may be installed in large facilities, applied as one phase AC current at 600 to 2000 Hz and peak voltages between 3000 and 20000 volts.
The dominating parameter influencing ozone generation efficiency is the gas temperature, which is controlled by the cooling water temperature. The cooler the water, the better the ozone synthesis. At typical industrial conditions, almost 90 percent of the effective power is dissipated as heat and needs to be removed by a sufficient cooling water flow.
Due to the high reactivity of ozone, only few materials may be used like stainless steel (quality 316L), glass, polytetrafluorethylene, or polyvinylidene fluoride. Viton may be used with the restriction of constant mechanical forces and absence of humidity.
Incidental production
Ozone may be formed from O2 by electrical discharges and by action of high energy electromagnetic radiation. Certain electrical equipment generate significant levels of ozone. This is especially true of devices using high voltages, such as ionic air purifiers, laser printers, photocopiers, and arc welders. Electric motors using brushes can generate ozone from repeated sparking inside the unit. Large motors that use brushes, such as those used by elevators or hydraulic pumps, will generate more ozone than smaller motors.
Laboratory production
In the laboratory ozone can be produced by electrolysis using a 9 volt battery, a pencil graphite rod cathode, a platinum wire anode and a 3M sulfuric acid electrolyte. The half cell reactions taking place are
- 3 H2O → O3 + 6 H+ + 6 e−; ΔEo = −1.53 V;
- 6 H+ + 6 e− → 3 H2; ΔEo = 0 V;
- 2 H2O → O2 + 4 H+ + 4 e−; ΔEo = −1.23 V;
so that in the net reaction three equivalents of water are converted into one equivalent of ozone and three equivalents of hydrogen. Oxygen formation is a competing reaction.
It can also be prepared by passing 10,000-20,000 volts DC through dry O2. This can be done with an apparatus consisting of two concentric glass tubes sealed together at the top, with in and and out spigots at the top and bottom of the outer tube. The inner core should have a length of metal foil inserted into it connected to one side of the power source. The other side of the power source should be connected to another piece of foil wrapped around the outer tube. Dry O2 should be run through the tube in one spigot. As the O2 is run through one spigot into the apparatus and 10,000-20,000 volts DC are applied to the foil leads, electricity will discharge between the dry dioxygen in the middle and form O3 in O2 out the other spigot. The reaction can be summarized as follows:
- 3 O2 — electricity → 2 O3
Applications
Industrial applications
At present, the uses of ozone as an industrial chemical are somewhat limited. The largest use of ozone is in the preparation of pharmaceuticals, synthetic lubricants, as well as many other commercially useful organic compounds, where it is used to sever carbon-carbon bonds. It can also be used for bleaching substances and for killing microorganisms in air and water sources. Many municipal drinking water systems kill bacteria with ozone instead of the more common chlorine. Ozone has a very high oxidation potential. Ozone does not form organochlorine compounds, nor does it remain in the water after treatment, so some systems introduce a small amount of chlorine to prevent bacterial growth in the pipes, or may use chlorine intermittently, based on results of periodic testing. Where electrical power is abundant, ozone is a cost-effective method of treating water, as it is produced on demand and does not require transportation and storage of hazardous chemicals. Once it has decayed, it leaves no taste or odour in drinking water. Low levels of ozone have been advertised to be of some disinfectant use in residential homes, however, the concentration of ozone required to have a substantial effect on airborne pathogens greatly exceeds safe levels recommended by the U.S. Occupational Safety and Health Administration and Environmental Protection Agency.
Industrially, ozone is used to:
- Disinfect laundry in hospitals, food factories, care homes etc;
- Water disinfectant in place of chlorine
- Deodorize air and objects, such as after a fire. This process is extensively used in Fabric Restoration;
- Kill bacteria on food or on contact surfaces;
- Ozone swimming pool and spa sanitation
- Scrub yeast and mold spores from the air in food processing plants;
- Wash fresh fruits and vegetables to kill yeast, mold and bacteria;
- Chemically attack contaminants in water (iron, arsenic, hydrogen sulfide, nitrites, and complex organics lumped together as "colour");
- Provide an aid to flocculation (agglomeration of molecules, which aids in filtration, where the iron and arsenic are removed);
- Manufacture chemical compounds via chemical synthesis
- Clean and bleach fabrics (the former use is utilized in Fabric Restoration)(the latter use is patented);
- Assist in processing plastics to allow adhesion of inks;
- Age rubber samples to determine the useful life of a batch of rubber;
- Hospital operating rooms where air needs to be sterile;
- Eradicate water borne parasites such as Giardia and Cryptosporidium in surface water treatment plants.
Ozone is a reagent in many organic reactions in the laboratory and in industry. Ozonolysis is the cleavage of an alkene to carbonyl compounds.
Many hospitals in the U.S. and around the world use large ozone generators to decontaminate operating rooms between surgeries. The rooms are cleaned and then sealed airtight before being filled with ozone which effectively kills or neutralizes all remaining bacteria.
Ozone is used as an alternative to chlorine or chlorine dioxide in the bleaching of wood pulp . It is often used in conjunction with oxygen and hydrogen peroxide to completely eliminate the need for chlorine-containing compounds in the manufacture of high-quality, white paper
Ozone can be used to detoxify cyanide wastes (for example from gold and silver mining) by oxidizing cyanide to cyanate and eventually to carbon dioxide.
Consumer applications
Devices generating high levels of ozone, some of which use ionization, are used to sanitize and deodorize uninhabited buildings, rooms, ductwork, woodsheds, and boats and other vehicles.
In the US, air purifiers emitting lower levels of ozone have been sold. This kind of air purifier is sometimes claimed to imitate nature's way of purifying the air without filters and to sanitize both it and household surfaces. The United States Environmental Protection Agency has declared that there is "evidence to show that at concentrations that do not exceed public health standards, ozone is not effective at removing many odour-causing chemicals" or "viruses, bacteria, mold, or other biological pollutants." Furthermore, its report states that "results of some controlled studies show that concentrations of ozone considerably higher than these [human safety] standards are possible even when a user follows the manufacturer’s operating instructions." The government successfully sued one company in 1995, ordering it to stop repeating health claims without supporting scientific studies.
Ozonated water is used to launder clothes and to sanitize food, drinking water, and surfaces in the home. According to the FDA, it is "amending the food additive regulations to provide for the safe use of ozone in gaseous and aqueous phases as an antimicrobial agent on food, including meat and poultry." Studies at California Polytechnic University demonstrated that 0.3 ppm levels of ozone dissolved in filtered tapwater can produce a reduction of more than 99.99% in such food-borne microorganisms as salmonella, E. coli 0157:H7, and Campylobacter. Ozone can be used to remove pesticide residues from fruits and vegetables.
New, patented technology uses ozone to disinfect and deodorize protective sports gear for football, hockey, and lacrosse by blowing it directly into the equipment to destroy bacteria within the padding. This has proven particularly useful in battling the spread of MRSA.
Ozone is used in spas and hot tubs to kill bacteria in the water and to reduce the amount of chlorine or bromine required by reactivating them to their free state. As ozone does not remain in the water long enough, ozone by itself is ineffective at preventing cross-contamination among bathers and must be used in conjunction with these halogens. Gaseous ozone created by ultraviolet light or by corona discharge is injected into the water.
Ozone is also widely used in treatment of water in aquariums and fish ponds. Its use can minimize bacterial growth, control parasites, eliminate transmission of some diseases, and reduce or eliminate "yellowing" of the water. Ozone must not come in contact with fish's gill structures. Natural salt water (with life forms) provides enough "instantaneous demand" that controlled amounts of ozone activate bromide ion to hypobromous acid, and the ozone entirely decays in a few seconds to minutes. If oxygen fed ozone is used, the water will be higher in dissolved oxygen, fish's gill structures will atrophy and they will become dependent on higher dissolved oxygen levels. The higher dissolved oxygen levels tend to minimize algal growth.
Ozone therapy
Ozone therapy has been used in alternative medicine as a medical treatment in a number of different countries. Its evidence base, however has been questioned by western medicine.
The United States Food and Drug Administration (FDA) based on the known toxic effects of ozone and the lack of scientific evidence of any beneficial effects at non-toxic levels, has a long established policy of prohibiting ozone generators or ozone gas from being marketed as a treatment for any medical conditions Ozone therapy is a well established alternative and complementary therapy in most mainland European countries where health authorities have tolerated its practice. The European Cooperation of Medical Ozone Societies, founded in 1972 publishes guidelines on medical indications and contraindications of ozone and hosts training seminars.
A significant amount of research has in recent years been published in peer reviewed journals of International Medical Societies that conficts with the views of the FDA. Modern medical applications of blood ozonation outside the body, conducted by Celacade and EBOO have recently been found the therapy in clinical trials to be safe and effective.