Atom
Background Information
SOS Children volunteers helped choose articles and made other curriculum material Sponsor a child to make a real difference.
| Helium atom | ||||||||
|---|---|---|---|---|---|---|---|---|
 |
||||||||
| An illustration of the helium atom, depicting the nucleus (pink) and the electron cloud distribution (black). The nucleus (upper right) is in reality spherically symmetric, although for more complicated nuclei this is not always the case. The black bar is one ångström, equal to 10−10 m or 100,000 fm. | ||||||||
| Classification | ||||||||
|
||||||||
| Properties | ||||||||
|
An atom is the smallest particle that comprises a chemical element. An atom consists of an electron cloud that surrounds a dense nucleus. This nucleus contains positively charged protons and electrically neutral neutrons, whereas the surrounding cloud is made up of negatively charged electrons. When the number of protons in the nucleus equals the number of electrons, the atom is electrically neutral; otherwise it is an ion and has a net positive or negative charge. An atom is classified according to its number of protons and neutrons: the number of protons determines the chemical element and the number of neutrons determines the isotope of that element. The concept of the atom as an indivisible component of matter was first proposed by early Indian and Greek philosophers. In the 17th and 18th centuries, chemists provided a physical basis for this idea by showing that certain substances could not be further broken down by chemical methods. During the late 19th and the early 20th centuries, physicists discovered subatomic components and structure inside the atom, thereby demonstrating that the 'atom' was not indivisible. The principles of quantum mechanics, including the wave–particle duality of matter, were used to successfully model the atom.
Relative to everyday experience, atoms are minuscule objects with proportionately tiny masses that can only be observed individually using special instruments such as the scanning tunneling microscope. More than 99.9% of an atom's mass is concentrated in the nucleus, with protons and neutrons having about equal mass. In atoms with too many or too few neutrons relative to the number of protons, the nucleus is unstable and subject to radioactive decay. The electrons surrounding the nucleus occupy a set of stable energy levels, or orbitals, and they can transition between these states by the absorption or emission of photons that match the energy differences between the levels. The electrons determine the chemical properties of an element, and strongly influence an atom's magnetic properties.
History
The concept that matter is composed of discrete units and cannot be divided into arbitrarily tiny quantities has been around for millennia, but these ideas were founded in abstract, philosophical reasoning rather than experimentation and empirical observation. The nature of atoms in philosophy varied considerably over time and between cultures and schools, and often had spiritual elements. Nevertheless, the basic idea of the atom was adopted by scientists thousands of years later because it elegantly explained new discoveries in the field of chemistry.
The earliest references to the concept of atoms date back to ancient India in the 6th century BCE. The Nyaya and Vaisheshika schools developed elaborate theories of how atoms combined into more complex objects (first in pairs, then trios of pairs). The references to atoms in the West emerged a century later from Leucippus whose student, Democritus, systemized his views. In approximately 450 BCE, Democritus coined the term átomos (Greek ἄτομος), which meant "uncuttable" or "the smallest indivisible particle of matter", i.e., something that cannot be divided. Although the Indian and Greek concepts of the atom were based purely on philosophy, modern science has retained the name coined by Democritus.
Further progress in the understanding of atoms did not occur until the science of chemistry began to develop. In 1661, the natural philosopher Robert Boyle published The Sceptical Chymist in which he argued that matter was composed of various combinations of different "corpuscules" or atoms, rather than the classical elements of air, earth, fire and water. In 1789 the term element was defined by the French nobleman and scientific researcher Antoine Lavoisier to mean basic substances that could not be further broken down by the methods of chemistry.

In 1803, the Englishman John Dalton, an instructor and natural philosopher, used the concept of atoms to explain why elements always reacted in a ratio of small whole numbers—the law of multiple proportions—and why certain gases dissolved better in water than others. He proposed that each element consists of atoms of a single, unique type, and that these atoms could join to each other, to form chemical compounds.
Additional validation of particle theory (and by extension atomic theory) occurred in 1827 when botanist Robert Brown used a microscope to look at dust grains floating in water and discovered that they moved about erratically—a phenomenon that became known as " Brownian motion". J. Desaulx suggested in 1877 that the phenomenon was caused by the thermal motion of water molecules, and in 1905 Albert Einstein produced the first mathematical analysis of the motion, thus confirming the hypothesis.
The physicist J. J. Thomson, through his work on cathode rays in 1897, discovered the electron and its subatomic nature, which destroyed the concept of atoms as being indivisible units. Thomson believed that the electrons were distributed throughout the atom, with their charge balanced by the presence of a uniform sea of positive charge (the plum pudding model).
However, in 1909, researchers under the direction of physicist Ernest Rutherford bombarded a sheet of gold foil with helium ions and discovered that a small percentage were deflected through much larger angles than was predicted using Thomson's proposal. Rutherford interpreted the gold foil experiment as suggesting that the positive charge of an atom and most of its mass was concentrated in a nucleus at the centre of the atom (the Rutherford model), with the electrons orbiting it like planets around a sun. Positively charged helium ions passing close to this dense nucleus would then be deflected away at much sharper angles.
While experimenting with the products of radioactive decay, in 1913 radiochemist Frederick Soddy discovered that there appeared to be more than one type of atom at each position on the periodic table. The term isotope was coined by Margaret Todd as a suitable name for different atoms that belong to the same element. J.J. Thomson created a technique for separating atom types through his work on ionized gases, which subsequently led to the discovery of stable isotopes.
Meanwhile, in 1913, physicist Niels Bohr revised Rutherford's model by suggesting that the electrons were confined into clearly defined orbits, and could jump between these, but could not freely spiral inward or outward in intermediate states. An electron must absorb or emit specific amounts of energy to transition between these fixed orbits. When the light from a heated material is passed through a prism, it produced a multi-colored spectrum. The appearance of fixed lines in this spectrum was successfully explained by the orbital transitions.
In 1926, Erwin Schrödinger, using Louis de Broglie's 1924 proposal that particles behave to an extent like waves, developed a mathematical model of the atom that described the electrons as three-dimensional waveforms, rather than point particles. A consequence of using waveforms to describe electrons is that it is mathematically impossible to obtain precise values for both the position and momentum of a particle at the same time; this became known as the uncertainty principle. In this concept, for each measurement of a position one could only obtain a range of probable values for momentum, and vice versa. Although this model was difficult to visually conceptualize, it was able to explain observations of atomic behaviour that previous models could not, such as certain structural and spectral patterns of atoms larger than hydrogen. Thus, the planetary model of the atom was discarded in favour of one that described orbital zones around the nucleus where a given electron is most likely to exist.
The development of the mass spectrometer allowed the exact mass of atoms to be measured. The device uses a magnet to bend the trajectory of a beam of ions, and the amount of deflection is determined by the ratio of an atom's mass to its charge. The chemist Francis William Aston used this instrument to demonstrate that isotopes had different masses. The mass of these isotopes varied by integer amounts, called the whole number rule. The explanation for these different atomic isotopes awaited the discovery of the neutron, a neutral-charged particle with a mass similar to the proton, by the physicist James Chadwick in 1932. Isotopes were then explained as elements with the same number of protons, but different numbers of neutrons within the nucleus.
In the 1950s, the development of improved particle accelerator and particle detectors allowed scientists to study the impacts of atoms moving at high energies. Neutrons and protons were found to be hadrons, or composites of smaller particles called quarks. Standard models of nuclear physics were developed that successfully explained the properties of the nucleus in terms of these sub-atomic particles and the forces that govern their interactions.
Around 1985, Steven Chu and co-workers at Bell Labs developed a technique for lowering the temperatures of atoms using lasers. In the same year, a team led by William D. Phillips managed to contain atoms of sodium in a magnetic trap. The combination of these two techniques and a method based on the Doppler effect, developed by Claude Cohen-Tannoudji and his group, allows small numbers of atoms to be cooled to several microkelvin. This allows the atoms to be studied with great precision, and later led to the discovery of Bose-Einstein condensation.
Historically, single atoms have been prohibitively small for scientific applications. Recently, devices have been constructed that use a single metal atom connected through organic ligands to construct a single electron transistor. Experiments have been carried out by trapping and slowing single atoms using laser cooling in a cavity to gain a better physical understanding of matter.
Components
Subatomic particles
Though the word atom originally denoted a particle that cannot be cut into smaller particles, in modern scientific usage the atom is composed of various subatomic particles. The constituent particles of an atom consist of the electron, the proton and, for atoms other than hydrogen-1, the neutron.
The electron is by far the least massive of these particles at 9.11×10−28 g, with a negative electrical charge and a size that is too small to be measured using available techniques. Protons have a positive charge and a mass 1,836 times that of the electron, at 1.6726×10−24 g, although this can be reduced by changes to the atomic binding energy. Neutrons have no electrical charge and have a free mass of 1,839 times the mass of electrons, or 1.6929×10−24 g. Neutrons and protons have comparable dimensions—on the order of 2.5×10−15 m—although the 'surface' of these particles is not sharply defined.
In the Standard Model of physics, both protons and neutrons are composed of elementary particles called quarks. The quark is a type of fermion, one of the two basic constituents of matter—the other being the lepton, of which the electron is an example. There are six types of quarks, and each has a fractional electric charge of either +2/3 or −1/3. Protons are composed of two up quarks and one down quark, while a neutron consists of one up quark and two down quarks. This distinction accounts for the difference in mass and charge between the two particles. The quarks are held together by the strong nuclear force, which is mediated by gluons. The gluon is a member of the family of bosons, which are elementary particles that mediate physical forces.
Nucleus
All of the bound protons and neutrons in an atom make up a tiny atomic nucleus, and are collectively called nucleons. The radius of a nucleus is approximately equal to ![\begin{smallmatrix}1.07 \cdot \sqrt[3]{A}\end{smallmatrix}](../../images/170/17089.png) fm, where A is the total number of nucleons. This is much smaller than the radius of the atom, which is on the order of 105 fm. The nucleons are bound together by a short-ranged attractive potential called the residual strong force. At distances smaller than 2.5 fm, this force is much more powerful than the electrostatic force that causes positively charged protons to repel each other.
fm, where A is the total number of nucleons. This is much smaller than the radius of the atom, which is on the order of 105 fm. The nucleons are bound together by a short-ranged attractive potential called the residual strong force. At distances smaller than 2.5 fm, this force is much more powerful than the electrostatic force that causes positively charged protons to repel each other.
Atoms of the same element have the same number of protons, called the atomic number. Within a single element, the number of neutrons may vary, determining the isotope of that element. The number of neutrons relative to the protons determines the stability of the nucleus, with certain isotopes undergoing radioactive decay.
The neutron and the proton are different types of fermions. The Pauli exclusion principle is a quantum mechanical effect that prohibits identical fermions (such as multiple protons) from occupying the same quantum state at the same time. Thus every proton in the nucleus must occupy a different state, with its own energy level, and the same rule applies to all of the neutrons. (This prohibition does not apply to a proton and neutron occupying the same quantum state.)
A nucleus that has a different number of protons than neutrons can potentially drop to a lower energy state through a radioactive decay that causes the number of protons and neutrons to more closely match. As a result, atoms with matching numbers of protons and neutrons are more stable against decay. However, with increasing atomic number, the mutual repulsion of the protons requires an increasing proportion of neutrons to maintain the stability of the nucleus, which slightly modifies this trend of equal numbers of protons to neutrons.
The number of protons and neutrons in the atomic nucleus can be modified, although this can require very high energies because of the strong force. Nuclear fusion occurs when multiple atomic particles join to form a heavier nucleus, such as through the energetic collision of two nuclei. At the core of the Sun, protons require energies of 3–10 KeV to overcome their mutual repulsion—the coulomb barrier—and fuse together into a single nucleus. Nuclear fission is the opposite process, causing a nucleus to split into two smaller nuclei—usually through radioactive decay. The nucleus can also be modified through bombardment by high energy subatomic particles or photons. In such processes that change the number of protons in a nucleus, the atom becomes an atom of a different chemical element.
The mass of the nucleus following a fusion reaction is less than the sum of the masses of the separate particles. The difference between these two values is emitted as energy, as described by Albert Einstein's mass–energy equivalence formula, E = mc², where m is the mass loss and c is the speed of light. This deficit is the binding energy of the nucleus.
The fusion of two nuclei that have lower atomic numbers than iron and nickel is an exothermic process that releases more energy than is required to bring them together. It is this energy-releasing process that makes nuclear fusion in stars a self-sustaining reaction. For heavier nuclei, the total binding energy begins to decrease. That means fusion processes with nuclei that have higher atomic numbers is an endothermic process. These more massive nuclei can not undergo an energy-producing fusion reaction that can sustain the hydrostatic equilibrium of a star.
Electron cloud
The electrons in an atom are attracted to the protons in the nucleus by the electromagnetic force. This force binds the electrons inside an electrostatic potential well surrounding the smaller nucleus, which means that an external source of energy is needed in order for the electron to escape. The closer an electron is to the nucleus, the greater the attractive force. Hence electrons bound near the centre of the potential well require more energy to escape than those at the exterior.
Electrons, like other particles, have properties of both a particle and a wave. The electron cloud is a region inside the potential well where each electron forms a type of three-dimensional standing wave—a wave form that does not move relative to the nucleus. This behaviour is defined by an atomic orbital, a mathematical function that characterises the probability that an electron will appear to be at a particular location when its position is measured. Only a discrete (or quantized) set of these orbitals exist around the nucleus, as other possible wave patterns will rapidly decay into a more stable form. Orbitals can have one or more ring or node structures, and they differ from each other in size, shape and orientation.
Each atomic orbital corresponds to a particular energy level of the electron. The electron can change its state to a higher energy level by absorbing a photon with sufficient energy to boost it into the new quantum state. Likewise, through spontaneous emission, an electron in a higher energy state can drop to a lower energy state while radiating the excess energy as a photon. These characteristic energy values, defined by the differences in the energies of the quantum states, are responsible for atomic spectral lines.
The amount of energy needed to remove or add an electron (the electron binding energy) is far less than the binding energy of nucleons. For example, it requires only 13.6 eV to strip a ground-state electron from a Hydrogen atom. Atoms are electrically neutral if they have an equal number of protons and electrons. Atoms that have either a deficit or a surplus of electrons are called ions. Electrons that are farthest from the nucleus may be transferred to other nearby atoms or shared between atoms. By this mechanism, atoms are able to bond into molecules and other types of chemical compounds like ionic and covalent network crystals.
Properties
By definition, any two atoms with an identical number of protons in their nuclei belong to the same chemical element. Atoms with the same number of protons but a different number of neutrons are different isotopes of the same element. Hydrogen atoms, for example, always have only a single proton, but isotopes exist with no neutrons ( hydrogen-1, sometimes called protium, by far the most common form), one neutron ( deuterium) and two neutrons ( tritium). The known elements form a continuous range of atomic numbers from hydrogen with a single proton up to the 118-proton element ununoctium. All known isotopes of elements with atomic numbers greater than 82 are radioactive.
Mass
Because the large majority of an atom's mass comes from the protons and neutrons, the total number of these particles in an atom is called the mass number. The mass of an atom at rest is often expressed using the unified atomic mass unit (u), which is also called a Dalton (Da). This unit is defined as a twelfth of the mass of a free neutral atom of carbon-12, which is approximately 1.66×10−24 g. hydrogen-1, the lightest isotope of hydrogen and the atom with the lowest mass, has an atomic weight of 1.007825 u. An atom has a mass approximately equal to the mass number times the atomic mass unit. The heaviest stable atom is lead-208, with a mass of 207.9766521 u.
As even the most massive atoms are far too light to work with directly, chemists instead use the unit of moles. The mole is defined such that one mole of any element will always have the same number of atoms (about 6.022×1023). This number was chosen so that if an element has an atomic mass of 1 u, a mole of atoms of that element will have a mass of 1 g. Carbon, for example, has an atomic mass of 12 u, so a mole of carbon atoms weighs 12 g.
Size
Atoms lack a well-defined outer boundary, so the dimensions are usually described in terms of the distances between two nuclei when the two atoms are joined in a chemical bond. The radius varies with the location of an atom on the atomic chart, the type of chemical bond, the number of neighboring atoms ( coordination number) and a quantum mechanical property known as spin. On the periodic table of the elements, atom size tends to increase when moving down columns, but decrease when moving across rows (left to right). Consequently, the smallest atom is helium with a radius of 32 pm, while one of the largest is caesium at 225 pm. These dimensions are thousands of times smaller than the wavelengths of light (400–700 nm) so they can not be viewed using an optical microscope. However, individual atoms can be observed using a scanning tunneling microscope.
Some examples will demonstrate the minuteness of the atom. A typical human hair is about 1 million carbon atoms in width. A single drop of water contains about 2 sextillion (2×1021) atoms of oxygen, and twice the number of hydrogen atoms. A single carat diamond with a mass of 0.2 g contains about 10 sextillion atoms of carbon. If an apple was magnified to the size of the Earth, then the atoms in the apple would be approximately the size of the original apple.
Radioactive decay
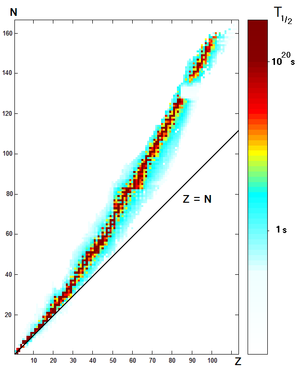
Every element has one or more isotopes that have unstable nuclei that are subject to radioactive decay, causing the nucleus to emit particles or electromagnetic radiation. Radioactivity can occur when the radius of a nucleus is large compared with the radius of the strong force, which only acts over distances on the order of 1 fm.
There are three primary forms of radioactive decay:
- Alpha decay is caused when the nucleus emits an alpha particle, which is a helium nucleus consisting of two protons and two neutrons. The result of the emission is a new element with a lower atomic number.
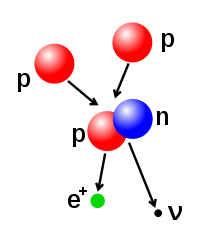
- Beta decay is regulated by the weak force, and results from a transformation of a neutron into a proton, or a proton into a neutron. The first is accompanied by the emission of an electron and an antineutrino, while the second causes the emission of a positron and a neutrino. The electron or positron emissions are called beta particles. Beta decay either increases or decreases the atomic number of the nucleus by one.
- Gamma decay results from a change in the energy level of the nucleus to a lower state, resulting in the emission of electromagnetic radiation. This can occur following the emission of an alpha or a beta particle from radioactive decay.
Each radioactive isotope has a characteristic decay time period—the half-life—that is determined by the amount of time needed for half of a sample to decay. This is an exponential decay process that steadily decreases the proportion of the remaining isotope by 50% every half life. Hence after two half-lives have passed only 25% of the isotope will be present, and so forth.
Magnetic moment
Elementary particles possess an intrinsic quantum mechanical property known as spin. This is analogous to the angular momentum of an object that is spinning around its centre of mass, although strictly speaking these particles are believed to be point-like and cannot be said to be rotating. Spin is measured in units of the reduced Planck constant (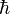 ), with electrons, protons and neutrons all having spin ½
), with electrons, protons and neutrons all having spin ½ 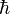 , or "spin-½". In an atom, electrons in motion around the nucleus possess orbital angular momentum in addition to their spin, while the nucleus itself possesses angular momentum due to its nuclear spin.
, or "spin-½". In an atom, electrons in motion around the nucleus possess orbital angular momentum in addition to their spin, while the nucleus itself possesses angular momentum due to its nuclear spin.
The magnetic field produced by an atom—its magnetic moment—is determined by these various forms of angular momentum, just as a rotating charged object classically produces a magnetic field. However, the most dominant contribution comes from spin. Due to the nature of electrons to obey the Pauli exclusion principle, in which no two electrons may be found in the same quantum state, bound electrons pair up with each other, with one member of each pair in a spin up state and the other in the opposite, spin down state. Thus these spins cancel each other out, reducing the total magnetic dipole moment to zero in some atoms with even number of electrons.
In ferromagnetic elements such as iron, an odd number of electrons leads to an unpaired electron and a net overall magnetic moment. The orbitals of neighboring atoms overlap and a lower energy state is achieved when the spins of unpaired electrons are aligned with each other, a process is known as an exchange interaction. When the magnetic moments of ferromagnetic atoms are lined up, the material can produce a measurable macroscopic field. Paramagnetic materials have atoms with magnetic moments that line up in random directions when no magnetic field is present, but the magnetic moments of the individual atoms line up in the presence of a field.
The nucleus of an atom can also have a net spin. Normally these nuclei are aligned in random directions because of thermal equilibrium. However, for certain elements (such as xenon-129) it is possible to polarize a significant proportion of the nuclear spin states so that they are aligned in the same direction—a condition called hyperpolarization. This has important applications in magnetic resonance imaging.
Energy levels
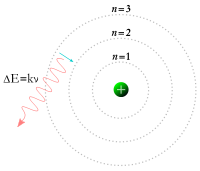
When an electron is bound to an atom, it has a potential energy that is inversely proportional to its distance from the nucleus. This is measured by the amount of energy needed to unbind the electron from the atom, and is usually given in units of electronvolts (eV). In the quantum mechanical model, a bound electron can only occupy a set of states centered on the nucleus, and each state corresponds to a specific energy level. The lowest energy state of a bound electron is called the ground state, while an electron at a higher energy level is in an excited state.
In order for an electron to transition between two different states, it must absorb or emit a photon at an energy matching the difference in the potential energy of those levels. The energy of an emitted photon is proportional to its frequency, so these specific energy levels appear as distinct bands in the electromagnetic spectrum. Each element has a characteristic spectrum that can depend on the nuclear charge, subshells filled by electrons, the electromagnetic interactions between the electrons and other factors.
When a continuous spectrum of energy is passed through a gas or plasma, some of the photons are absorbed by atoms, causing electrons to change their energy level. Those excited electrons that remain bound to their atom will spontaneously emit this energy as a photon, traveling in a random direction, and so drop back to lower energy levels. Thus the atoms behave like a filter that forms a series of dark absorption bands in the energy output. (An observer viewing the atoms from a different direction, which does not include the continuous spectrum in the background, will instead see a series of emission lines from the photons emitted by the atoms.) Spectroscopic measurements of the strength and width of spectral lines allow the composition and physical properties of a substance to be determined.
Close examination of the spectral lines reveals that some display a fine structure splitting. This occurs because of spin-orbit coupling, which is an interaction between the spin and motion of the outermost electron. When an atom is in an external magnetic field, spectral lines become split into three or more components; a phenomenon called the Zeeman effect. This is caused by the interaction of the magnetic field with the magnetic moment of the atom and its electrons. Some atoms can have multiple electron configurations with the same energy level, which thus appear as a single spectral line. The interaction of the magnetic field with the atom shifts these electron configurations to slightly different energy levels, resulting in multiple spectral lines. The presence of an external electric field can cause a comparable splitting and shifting of spectral lines by modifying the electron energy levels, a phenomenon called the Stark effect.
If a bound electron is in an excited state, an interacting photon with the proper energy can cause stimulated emission of a photon with a matching energy level. For this to occur, the electron must drop to a lower energy state that has an energy difference matching the energy of the interacting photon. The emitted photon and the interacting photon will then move off in parallel and with matching phases. That is, the wave patterns of the two photons will be synchronized. This physical property is used to make lasers, which can emit a coherent beam of light energy in a narrow frequency band.
Valence
The outermost electron shell of an atom in its uncombined state is known as the valence shell, and the electrons in that shell are called valence electrons. The number of valence electrons determines the bonding behaviour with other atoms. Atoms tend to chemically react with each other in a manner that will fill (or empty) their outer valence shells.
The chemical elements are often displayed in a periodic table that is laid out to display recurring chemical properties, and elements with the same number of valence electrons form a group that is aligned in the same column of the table. (The horizontal rows correspond to the filling of a quantum shell of electrons.) The elements at the far right of the table have their outer shell completely filled with electrons, which results in chemically inert elements known as the noble gases.
States
Quantities of atoms are found in different states of matter that depend on the physical conditions, such as temperature and pressure. By varying the conditions, materials can transition between solids, liquids, gases and plasmas. Within a state, a material can also exist in different phases. An example of this is solid carbon, which can exist as graphite or diamond.
At temperatures close to absolute zero, atoms can form a Bose–Einstein condensate, at which point quantum mechanical effects, which are normally only observed at the atomic scale, become apparent on a macroscopic scale. This super-cooled collection of atoms then behaves as a single Super Atom, which may allow fundamental checks of quantum mechanical behaviour.
Identification
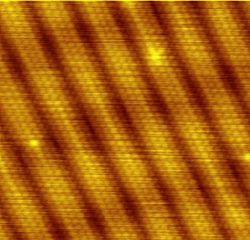
The scanning tunneling microscope is a device for viewing surfaces at the atomic level. It uses the quantum tunneling phenomenon, which allows particles to pass through a barrier that would normally be insurmountable. Electrons tunnel through the vacuum between two planar metal electrodes, on each of which is an adsorbed atom, providing a tunneling-current density that can be measured. Scanning one atom (taken as the tip) as it moves past the other (the sample) permits plotting of tip displacement versus lateral separation for a constant current. The calculation shows the extent to which scanning-tunneling-microscope images of an individual atom are visible. It confirms that for low bias, the microscope images the space-averaged dimensions of the electron orbitals across closely packed energy levels—the Fermi level local density of states.
An atom can be ionized by removing one of its electrons. The electric charge causes the trajectory of an atom to bend when it passes through a magnetic field. The radius by which the trajectory of a moving ion is turned by the magnetic field is determined by the mass of the atom. The mass spectrometer uses this principle to measure the mass-to-charge ratio of ions. If a sample contains multiple isotopes, the mass spectrometer can determine the proportion of each isotope in the sample by measuring the intensity of the different beams of ions. Techniques to vaporize atoms include inductively coupled plasma atomic emission spectroscopy and inductively coupled plasma mass spectrometry, both of which use a plasma to vaporize samples for analysis.
A more area-selective method is electron energy loss spectroscopy, which measures the energy loss of an electron beam within a transmission electron microscope when it interacts with a portion of a sample. The atom-probe tomograph has sub-nanometer resolution in 3-D and can chemically identify individual atoms using time-of-flight mass spectrometry.
Spectra of excited states can be used to analyze the atomic composition of distant stars. Specific light wavelengths contained in the observed light from stars can be separated out and related to the quantized transitions in free gas atoms. These colors can be replicated using a gas-discharge lamp containing the same element. Helium was discovered in this way in the spectrum of the Sun 23 years before it was found on Earth.
Origin and current state
Atoms form about 4% of the total mass density of the observable universe, with an average density of about 0.25 atoms/m3. Within a galaxy such as the Milky Way, atoms have a much higher concentration, with the density of matter in the interstellar medium (ISM) ranging from 105 to 109 atoms/m3. The Sun is believed to be inside the Local Bubble, a region of highly ionized gas, so the density in the solar neighbourhood is only about 103 atoms/m3. Stars form from dense clouds in the ISM, and the evolutionary processes of stars result in the steady enrichment of the ISM with elements more massive than hydrogen and helium. Up to 95% of the Milky Way's atoms are concentrated inside stars and the total mass of atoms forms about 10% of the mass of the galaxy. (The remainder of the mass is an unknown dark matter.)
Nucleosynthesis
Stable protons and electrons appeared one second after the Big Bang. During the following three minutes, Big Bang nucleosynthesis produced most of the helium, lithium, and deuterium atoms in the universe, and perhaps some of the beryllium and boron. The first atoms (complete with bound electrons) were theoretically created 380,000 years after the Big Bang—an epoch called recombination, when the expanding universe cooled enough to allow electrons to become attached to nuclei. Since then, atomic nuclei have been combined in stars through the process of nuclear fusion to produce elements up to iron.
Isotopes such as lithium-6 are generated in space through cosmic ray spallation. This occurs when a high-energy proton strikes an atomic nucleus, causing large numbers of nucleons to be ejected. Elements heavier than iron were produced in supernovae through the r-process and in AGB stars through the s-process, both of which involve the capture of neutrons by atomic nuclei. Elements such as lead formed largely through the radioactive decay of heavier elements.
Earth
Most of the atoms that make up the Earth and its inhabitants were present in their current form in the nebula that collapsed out of a molecular cloud to form the solar system. The rest are the result of radioactive decay, and their relative proportion can be used to determine the age of the Earth through radiometric dating. Most of the helium in the crust of the Earth (about 99% of the helium from gas wells, as shown by its lower abundance of helium-3) is a product of alpha decay.
There are a few trace atoms on Earth that were not present at the beginning (i.e., not "primordial"), nor are results of radioactive decay. Carbon-14 is continuously generated by cosmic rays in the atmosphere. Some atoms on Earth have been artificially generated either deliberately or as by-products of nuclear reactors or explosions. Of the transuranic elements—those with atomic numbers greater than 92—only plutonium and neptunium occur naturally on Earth. Transuranic elements have radioactive lifetimes shorter than the current age of the Earth and thus identifiable quantities of these elements have long since decayed, with the exception of traces of plutonium-244 possibly deposited by cosmic dust. Natural deposits of plutonium and neptunium are produced by neutron capture in uranium ore.
The Earth contains approximately 1.33×1050 atoms. In the planet's atmosphere, small numbers of independent atoms exist for the noble gases, such as argon and neon. The remaining 99% of the atmosphere is bound in the form of molecules, including carbon dioxide and diatomic oxygen and nitrogen. At the surface of the Earth, atoms combine to form various compounds, including water, salt, silicates and oxides. Atoms can also combine to create materials that do not consist of discrete molecules, including crystals and liquid or solid metals. This atomic matter forms networked arrangements that lack the particular type of small-scale interrupted order associated with molecular matter.
Rare and theoretical forms
While isotopes with atomic numbers higher than lead (82) are known to be radioactive, an " island of stability" has been proposed for some elements with atomic numbers above 103. These superheavy elements may have a nucleus that is relatively stable against radioactive decay. The most likely candidate for a stable superheavy atom, unbihexium, has 126 protons and 184 neutrons.
Each particle of matter has a corresponding antimatter particle with the opposite electrical charge. Thus, the positron is a positively charged antielectron and the antiproton is a negatively charged equivalent of a proton. For unknown reasons, antimatter particles are rare in the universe, hence, no antimatter atoms have been discovered. Antihydrogen, the antimatter counterpart of hydrogen, was first produced at the CERN laboratory in Geneva in 1996.
Other exotic atoms have been created by replacing one of the protons, neutrons or electrons with other particles that have the same charge. For example, an electron can be replaced by a more massive muon, forming a muonic atom. These types of atoms can be used to test the fundamental predictions of physics.