A mammary gland is an organ in female mammals that produces milk to feed young offspring.
Anatomy of the Mammary Gland
The basic components of a mature mammary gland are the alveoli, hollow cavities, a few millimeters large lined with milk-secreting cuboidal cells and surrounded by myoepithelial cells. These alveoli join to form groups known as lobules, and each lobule has a lactiferous duct that drains into openings in the nipple. The myoepithelial cells can contract under the stimulation of oxytocin, excreting milk secreted from alveolar units into the lobule lumen toward the nipple where it collects in sinuses of the ducts. As the infant begins to suck, the hormonally (oxytocin) mediated "let-down reflex" ensues, and the mother's milk is secreted into the baby's mouth.
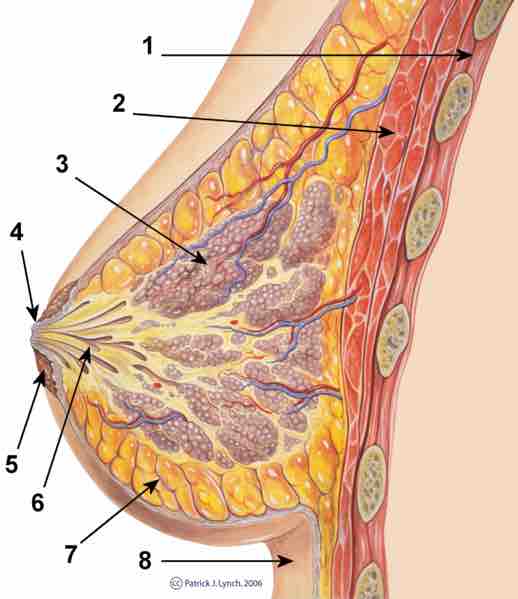
Mammary Gland
Cross-section of the mammary-gland. 1. Chest wall 2. Pectoralis muscles 3. Lobules 4. Nipple 5. Areola 6. Milk duct 7. Fatty tissue 8. Skin EndFragment
All the milk-secreting tissue leading to a single lactiferous duct is called a simple mammary gland; a complex mammary gland is all the simple mammary glands serving one nipple. Humans normally have two complex mammary glands, one in each breast, and each complex mammary gland consists of 10–20 simple glands. The presence of more than two nipples is known as polythelia, and the presence of more than two complex mammary glands as polymastia.
Development of the Mammary Glands
Mammary glands develop during different growth cycles. They exist in both sexes during the embryonic stage, forming only a rudimentary duct tree at birth. In this stage, mammary gland development depends on systemic (and maternal) hormones, but is also under the local regulation of paracrine communication between neighboring epithelial and mesenchymal cells by parathyroid hormone-related protein. This locally-secreted factor gives rise to a series of outside-in and inside-out positive feedback between these two types of cells, so that mammary bud epithelial cells can proliferate and sprout down into the mesenchymal layer until they reach the fat pad to begin the first round of branching.
Lactiferous duct development occurs in females in response to circulating hormones, first during pre- and postnatal stages and later during puberty. Estrogen promotes branching differentiation, which is inhibited by testosterone in males. A mature duct tree reaching the limit of the fat pad of the mammary gland is formed by bifurcation of duct terminal end buds, secondary branches sprouting from primary ducts and proper duct lumen formation.
The Process of Milk Production
Secretory alveoli develop mainly in pregnancy, when rising levels of prolactin, estrogen, and progesterone cause further branching, together with an increase in adipose tissue and a richer blood flow. In gestation, serum progesterone remains at a high concentration so signaling through its receptor is continuously activated. As one of the transcribed genes, Wnts secreted from mammary epithelial cells act paracrinely to induce branching of neighboring cells. When the lactiferous duct tree is almost ready, alveoli are differentiated from luminal epithelial cells and added at the end of each branch. In late pregnancy and for the first few days after giving birth, colostrum is secreted.
Milk secretion (lactation) begins a few days after birth, caused by reduction in circulating progesterone and the presence of prolactin, which mediates further alveologenesis and milk protein production and regulates osmotic balance and tight junction function. The binding of laminin and collagen in the myoepithelial basement membrane with beta-1 integrin on the epithelial surface insures correct placement of prolactin receptors on basal lateral side of alveoli cells and directional secretion of milk into lactiferous ducts. Suckling of the baby causes release of hormone oxytocin which stimulates contraction of the myoepithelial cells. With combined control from the extracellular matrix (ECM) and systemic hormones, milk secretion can be reciprocally amplified to provide enough nutrition for the baby.
During weaning, decreased prolactin, lack of mechanical stimulation through suckling, and changes in osmotic balance caused by milk stasis and leaking of tight junctions cause cessation of milk production. In some species there is complete or partial involution of alveolar structures after weaning; however, in humans there is only partial involution, which widely varies among individuals. Shrinkage of the mammary duct tree and ECM remodeling by various proteinase is under the control of somatostatin and other growth-inhibiting hormones and local factors. This structure change leads loose fat tissue to fill the empty space. However, a functional lactiferous duct tree can be reformed when a female is pregnant again.