Carbon Dioxide Transport
Carbon dioxide is the product of cellular respiration, and is transported from the cells of tissues in the body to the alveoli of the lungs through the bloodstream. Carbon dioxide is carried in the blood through three different ways.
Dissolved in the Plasma
About 5% of carbon dioxide is transported in the plasma of the blood as dissolved CO2 molecules that aren't bound to anything else. Carbon dioxide has a much higher solubility than oxygen, which explains why a relatively greater amount of carbon dioxide is dissolved in the plasma compared to oxygen.
Bound to Hemoglobin
While oxygen binds to the iron content in the heme of hemoglobin, carbon dioxide can bind to the amino acid chains on hemoglobin. When carbon dioxide clings to hemoglobin it forms carbanimohemoglobin.
About 10% of carbon dioxide in the human body is transported this way. Carbanimohemoglobin gives red blood cells a bluish color, which is one of the reasons why the veins that carry deoxygenated blood appear to be blue.
A property of hemoglobin called the Haldane effect states that deoxygenated blood has an increased capacity to carry carbon dioxide, while oxygenated blood has a decreased capacity to carry carbon dioxide.
This property means that hemoglobin will primarily carry oxygen in systemic circulation until it unloads that oxygen and is able to carry a relatively higher amount of carbon dioxide. This is due to deoxygenated blood's increased capacity to carry carbon dioxide, and from the carbon dioxide loaded from the tissues during tissue gas exchange.
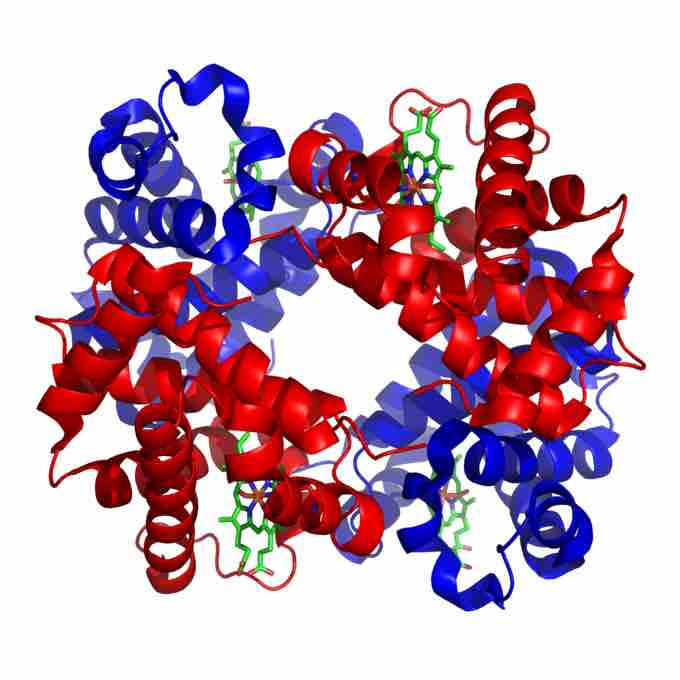
Structure of human hemoglobin
Hemoglobin is a tetramer of alpha (red) and beta (blue) subunits with iron containing heme groups (green).
Bicarbonate Ions
The majority (85%) of carbon dioxide travels in the blood stream as bicarbonate ions. The reaction that describes the formation of bicarbonate ions in the blood is:
CO2 + H2O → H2CO3 → H+ + HCO3–
This means that carbon dioxide reacts with water to form carbonic acid, which dissociates in solution to form hydrogen ions and bicarbonate ions.
The main implication of this process is that the pH of blood becomes a way of determining the amount of carbon dioxide in blood. This is because if carbon dioxide increases in the body, it will manifest as increased concentrations of bicarbonate and increased concentrations of hydrogen ions that reduce blood pH and make the blood more acidic.
Conversely, if carbon dioxide levels are reduced, there will be less bicarbonate and less hydrogen ions dissolved in the blood, so pH will increase and blood will become more basic. Bicarbonate ions act as a buffer for the pH of blood so that blood pH will be neutral as long as bicarbonate and hydrogen ions are balanced.
This connection explains how ventilation rate and blood chemistry are related, as hyperventilation will cause acidosis, and hypoventilation will cause alkalosis, due to the changes in carbon dioxide levels that they cause.
Bicarbonate is also carried in the fluids of tissues besides the blood vessels, especially in the duodenum and intestine, so problems in those organs can cause a respiratory system response.
Transport to the Alveoli
After carbon dioxide travels through the bloodstream to the capillaries covering the alveoli of the lungs through any of the 3 methods listed above, it must return to dissolved carbon dioxide form in order to diffuse across the capillary into the alveolus. Dissolved carbon dioxide is already able to diffuse into the alveolus, while hemoglobin-bound carbon dioxide is unloaded into the plasma.
For carbon dioxide stored in bicarbonate, it undergoes a reaction reversal. Bicarbonate ions dissolved in the plasma enter the red blood cells by diffusing across a chloride ion gradient (replacing chloride inside the cell), and combining with hydrogen to form carbonic acid.
Next, the action of carbonic anhydrase breaks carbonic acid down into carbon dioxide in water, which leaves the cell by diffusion. The dissolved carbon dioxide is then able to diffuse into the alveolus.