An antibody (formally called immunoglobulin) is a large Y-shaped glycoprotein produced by B-cells and used by the immune system to identify and neutralize pathogens. Antibodies are produced by B cells, and are either secreted into circulation or remain expressed on the surface of the B cell.
Structure of Antibodies
The antibody recognizes a unique part of an antigen (foreign object). Each tip of the "Y" of an antibody contains a paratope (a structure analogous to a lock) that is specific for one particular epitope (similarly analogous to a key) on an antigen, allowing these two structures to bind together with precision. Using this binding mechanism, an antibody can neutralize its target directly or tag it for attack by other parts of the immune system.
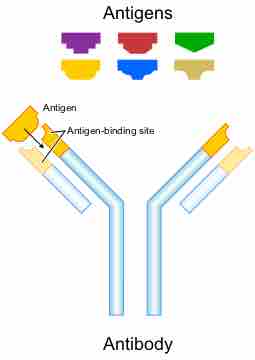
Antibody
Each antibody binds to a specific antigen, an interaction similar to a lock and key.
Antibodies are glycoproteins belonging to the immunoglobulin superfamily, typically made of basic structural units each with two large heavy chains and two small light chains. Most antibodies exist as a monomer, in which they have a single "Y" shaped sub-unit, but some antibodies can exist as dimers (two subunits) or pentamers (five subunits). The paratope is considered a hypervariable region and has the same specificity and antigen-binding affinity as the B cell receptor of the B cell that created the antibody. In some isotypes, the tail end of the antibody is called the constant region and faces away from the "Y-shaped" paratobe ends, functioning as an Fc tail to which phagocytes can bind.
Isotypes
Five different isotypes of antibodies each perform different functions and are generally found in different parts of the body.
- IgA: A dimer secreted into mucosal surfaces, such as the gut, respiratory tract, and urogenital tract, that prevents mucosal invasion into the body by pathogens. It is resistant to the proteolytic enzymes found in the gastrointestinal mucosae.
- IgD: Functions mainly as an antigen receptor on B cells that have not been exposed to antigens. It has been shown to activate basophils and mast cells to produce antimicrobial factors.
- IgE: Found in circulation and binds to allergens, triggering histamine release from mast cells and basophils. Also protects against parasitic worms.
- IgG: Has four different forms and provides the majority of antibody-based immunity against invading pathogens as the best opsonin of any type of antibody. This is because it expresses a tail for Fc receptors on phagocytes to bind to, which activates phagocytosis. It is the only antibody capable of crossing the placenta to give passive immunity to fetus, and can activate the classical complement system.
- IgM: Expressed on the surface of B cells (monomer) and in a secreted pentamer with very high avidity. Eliminates pathogens in the early stages of B cell-mediated (humoral) immunity before there is sufficient IgG. Like IgG, it can also activate the classical complement system.
Function of Antibodies
Circulating antibodies are produced by clonal B cells that specifically respond to only one antigen. Antibodies contribute to immunity in three ways: preventing pathogens from entering or damaging cells by binding to them (neutralization); stimulating removal of pathogens by macrophages and other cells by coating the pathogen (opsonization); and triggering destruction of pathogens by stimulating other immune responses such as the complement pathway. The complement system starts a long cascade of protein productions that either opsonize a pathogen for phagocytosis or lyse it directly by forming a membrane attack complex. During opsonization, the antibody expresses the tail for an Fc receptor on a macrophage, neutrophil, or natural killer cell. The immune cell will then bind to the antibody's Fc tail instead of the pathogen itself, which speeds up the process of finding pathogens to phagocytize. Additionally, because antibodies have two or more paratopes, they can sometimes link pathogens together, making phagocytosis more efficient.