Heat pumps, air conditioners, and refrigerators utilize heat transfer from cold to hot. Heat transfer (Qc) occurs from a cold reservoir and into a hot one. This requires work input W, which is also converted to heat transfer. Thus the heat transfer to the hot reservoir is Qh=Qc+W. A heat pump's mission is for heat transfer Qh to occur into a warm environment, such as a home in the winter. The mission of air conditioners and refrigerators is for heat transfer Qc to occur from a cool environment, such as chilling a room or keeping food at lower temperatures than the environment. Actually, a heat pump can be used both to heat and cool a space. It is essentially an air conditioner and a heating unit all in one. In this section we will concentrate on its heating mode.
Heat Pumps
A working fluid such as a non-CFC refrigerant is used in a basic heat pump. The basic components of a heat pump in are a condenser, an expansion valve, an evaporator and a compressor . In the outdoor coils (the evaporator), heat transfer Qc occurs to the working fluid from the cold outdoor air, turning it into a gas. The electrically driven compressor (work input W) raises the temperature and pressure of the gas and forces it into the condenser coils that are inside the heated space. Because the temperature of the gas is higher than the temperature inside the room, heat transfer to the room occurs and the gas condenses to a liquid. The liquid then flows back through a pressure-reducing valve to the outdoor evaporator coils, being cooled through expansion. (In a cooling cycle, the evaporator and condenser coils exchange roles and the flow direction of the fluid is reversed. )
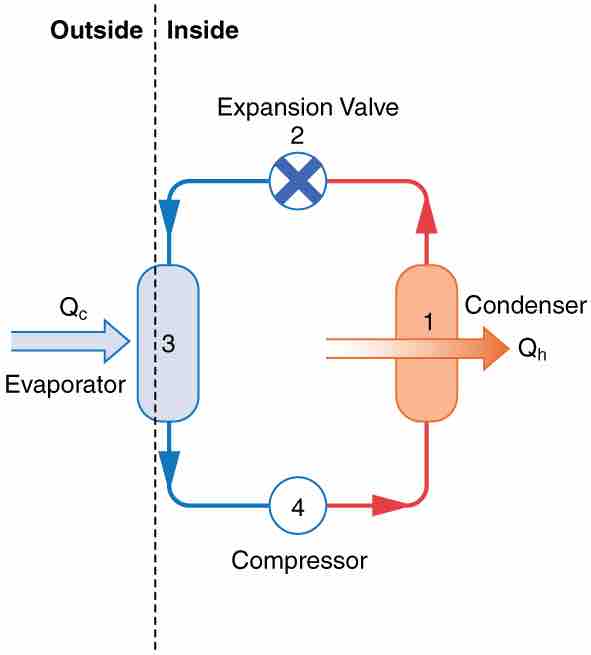
Simple Heat Pump
A simple heat pump has four basic components: (1) condenser, (2) expansion valve, (3) evaporator, and (4) compressor.
Coefficient of Performance
The quality of a heat pump is judged by how much heat transfer Qh occurs into the warm space compared with how much work input W is required. We define a heat pump's coefficient of performance (COPhp) to be
Since the efficiency of a heat engine is Eff=W/Qh, we see that COPhp=1/Eff. Since the efficiency of any heat engine is less than 1, it means that COPhp is always greater than 1—that is, a heat pump always has more heat transfer Qh than work put into it. Another interesting point is that heat pumps work best when temperature differences are small. The efficiency of a perfect engine (or Carnot engine) is
thus, the smaller the temperature difference, the smaller the efficiency and the greater the COPhp.
Air Conditioners and Refrigerators
Air conditioners and refrigerators are designed to cool something down in a warm environment. As with heat pumps, work input is required for heat transfer from cold to hot. The quality of air conditioners and refrigerators is judged by how much heat transfer Qc occurs from a cold environment compared with how much work input W is required. What is considered the benefit in a heat pump is considered waste heat in a refrigerator. We thus define the coefficient of performance (COPref) of an air conditioner or refrigerator to be
Since Qh=Qc+W and COPhp=Qh/W, we derive that
Also, from Qh>Qc, we see that an air conditioner will have a lower coefficient of performance than a heat pump.