What Are Sodium Pumps?
Na+/K+-ATPase (Sodium-potassium adenosine triphosphatase, also known as Na+/K+ pump, sodium-potassium pump, or sodium pump) is an antiporter enzyme (EC 3.6.3.9) (an electrogenic transmembrane ATPase) located in the plasma membrane of all animal cells.
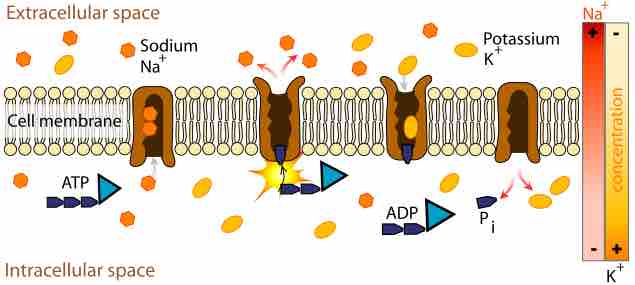
Active transport is responsible for cells containing relatively high concentrations of potassium ions but low concentrations of sodium ions. The mechanism responsible for this is the sodium-potassium pump, which moves these two ions in opposite directions across the plasma membrane. This was investigated by following the passage of radioactively labeled ions across the plasma membrane of certain cells. It was found that the concentrations of sodium and potassium ions on the two sides of the membrane are interdependent, suggesting that the same carrier transports both ions. It is now known that the carrier is an ATP-ase and that it pumps three sodium ions out of the cell for every two potassium ions pumped in.
Discovery and Significance
The sodium-potassium pump was discovered in the 1950s by Danish scientist Jens Christian Skou. It marked an important step in our understanding of how ions get into and out of cells, and has a particular significance for excitable cells like nervous cells, which depend on this pump for responding to stimuli and transmitting impulses.
The Na+/K+-ATPase helps maintain resting potential, avail transport and regulate cellular volume. It also functions as signal transducer/integrator to regulate MAPK pathway, ROS, as well as intracellular calcium. In most animal cells, the Na+/K+-ATPase is responsible for about 1/5 of the cell's energy expenditure. For neurons, the Na+/K+-ATPase can be responsible for up to 2/3 of the cell's energy expenditure.
Functions
Functions include resting potential, transport, controlling cell volume and acting as a signal transducer. The pump, while binding ATP, binds 3 intracellular Na+ ions. ATP is hydrolyzed, leading to phosphorylation of the pump at a highly conserved aspartate residue and subsequent release of ADP . A conformational change in the pump exposes the Na+ ions to the outside. The phosphorylated form of the pump has a low affinity for Na+ ions, so they are released.The pump binds 2 extracellular K+ ions. This causes the dephosphorylation of the pump, reverting it to its previous conformational state, transporting the K+ ions into the cell.The unphosphorylated form of the pump has a higher affinity for Na+ ions than K+ ions, so the two bound K+ ions are released. ATP binds, and the process starts again.
Na+/K+-ATPase
Na+/K+-ATPase Mechanism of Action
Proton Motive Force
Most bacteria rely on proton motive force as a source of energy for a variety of cellular processes. Usually, an H+ cycle includes generation of the transmembrane electrochemical gradient of H+ (proton motive force) by primary transport systems (H+ pumps) and its use for ATP synthesis, solute transport, motility and reverse electron transport. A substantial body of evidence indicates, however, that certain extremophilic bacteria can use Na+ as a coupling ion in an Na+ cycle instead of, or in addition to, the H+ cycle. As in the H+ cycle, a fully operational Na+ cycle would include a primary Na+ pump that directly couples Na+ translocation to a chemical reaction, an Na+-transporting membrane ATP synthetase, a number of Na+-dependent membrane transporters, and an Na+-dependent flagellar motor. While certain Na+-dependent functions, like Na+-dependent uptake of melibiose, proline, and glutamate, have been observed in many bacteria, the ion gradients that served as energy sources for these transports have been generated by primary H+ pumps and converted to Na+ gradients by Na+/H+ antiporters.
One could think of several possible explanations for the widespread distribution of the elements of the Na+ cycle among pathogenic bacteria. First, Na+-based membrane energetics could improve the versatility of a pathogen by providing it with additional means of ATP synthesis, motility and solute uptake. This would improve its chances for colonization of the host cells and survival in the host organisms where defense mechanisms, including generation of superoxide radicals, impair the integrity of the bacterial membrane and decrease the levels of the proton motive force. Second, because Na+ concentrations in most natural environments are almost 106-fold higher than H+ concentrations, sodium motive force levels are unlikely to change as rapidly as proton motive force levels, making sodium motive force a much more reliable source of energy.