Central to nitrogen fixation (N2 to NH3) are the enzymes that do the actual fixation, these are known as nitrogenases. Due to the oxidation carried out by oxygen, most nitrogenases, which are essential large reduction complexes are irreversibly inhibited by O2, which degradatively oxidizes the Fe-S cofactors. In essence, O2 binds to the iron (Fe) found in nitrogenases and blocks their ability to bind to N2. To protect nitrogenases, there are mechanisms for nitrogen fixers to protect nitrogenase from oxygen in vivo. One known exception is the nitrogenase of Streptomyces thermoautotrophicus, which is unaffected by the presence of oxygen. This is complicated by the fact the bacteria still need the presence of oxygen for proper respiration.
Some microbes have a proteoglycan rich extra cellular matrix which traps a layer of water, often referred to as a slime layer. This slime layer acts as a barrier for oxygen. The ability of some nitrogen fixers such as azotobacteraceae to employ an oxygen-amendable nitrogenase under aerobic conditions has been attributed to a high metabolic rate, allowing oxygen reduction at the cell membrane; however, the effectiveness of this mechanism is in question.
Many rhizobia, nitrogen fixing bacteria, live in a symbiotic relationship with plants known as legumes. They have an interesting strategy to deal with O2. In plants infected with Rhizobium, (legumes such as alfalfa or soybeans), the presence of oxygen in the root nodules would reduce the activity of the oxygen-sensitive nitrogenase. In these situations, the roots of such plants produce a protein known as leghemoglobin (also leghaemoglobin or legoglobin). Leghemoglobin buffers the concentration of free oxygen in the cytoplasm of infected plant cells to ensure the proper function of root nodules. Leghemoglobin is a nitrogen or oxygen carrier; naturally occurring oxygen and nitrogen interact similarly with this protein. Leghemoglobin buffers the concentration of free oxygen in the cytoplasm of infected plant cells to ensure the proper function of root nodules. It has close chemical and structural similarities to hemoglobin, and, like hemoglobin, is red in colour. Leghemoglobin has a high affinity for oxygen, about ten times higher than of human hemoglobin. This allows an oxygen concentration that is low enough to allow nitrogenase to function but not so high as to bind all the O2 in the bacteria, providing the bacteria with oxygen for respiration.
Leghemoglobin is produced by legumes in response to the roots being infected by rhizobia, as part of the symbiotic interaction between the plant and these nitrogen-fixing bacterium. Interestingly, it is widely believed that leghemoglobin is the product of both the plant and the bacterium in which a protein precursor is produced by the plant and the heme (an iron atom bound in a porphyrin ring, which binds O2) is produced by the bacterium. The protein and heme come together to function , allowing the bacteria to fix-nitrogen, giving the plant usable nitrogen and thus the plant provides the rhizobia a home.
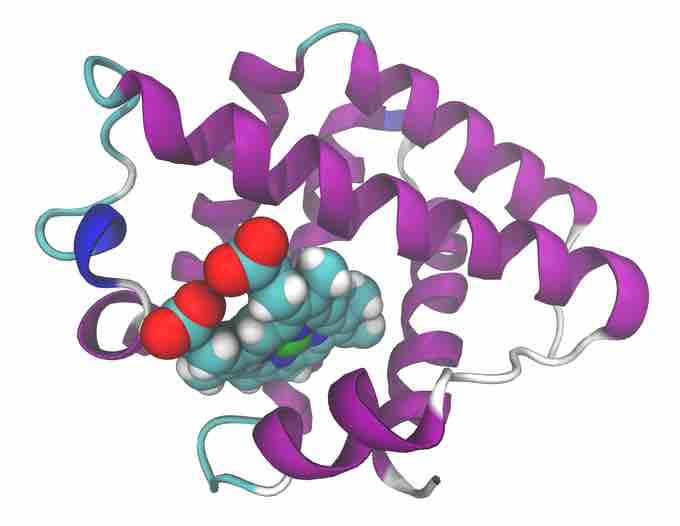
Leghemoglobin
Leghemoglobin, the protein which binds to oxygen, allowing nitrogenases to function in an oxygen free environment. The ribbons represent protein folds, while the conglomerate of spheres are the postion of the iron contain heme group which binds the oxygen.