Porphyrins are a group of organic compounds, many naturally occurring. One of the best-known porphyrins is heme, the pigment in red blood cells. Heme is a cofactor of the protein hemoglobin. The main "application" of porphyrins is their role in supporting aerobic life. For example, complexes of meso-tetraphenylporphyrin, e.g., the iron(III) chloride complex (TPPFeCl), catalyze a variety of reactions of potential interest in organic synthesis.
Porphyrins are heterocyclic macrocycles composed of four modified pyrrole subunits interconnected at their α carbon atoms via methine bridges (=CH-). Porphyrins are aromatic, obeying Hückel's rule for aromaticity, possessing 4n+2 π electrons (n=4 for the shortest cyclic path) delocalized over the macrocycle. Thus, porphyrin macrocycles are highly conjugated systems. As a consequence, they typically have very intense absorption bands in the visible region and may be deeply colored. (The name porphyrin comes from a Greek word for purple. ) The macrocycle has 26 pi electrons in total. The parent porphyrin is porphine, and substituted porphines are called porphyrins.
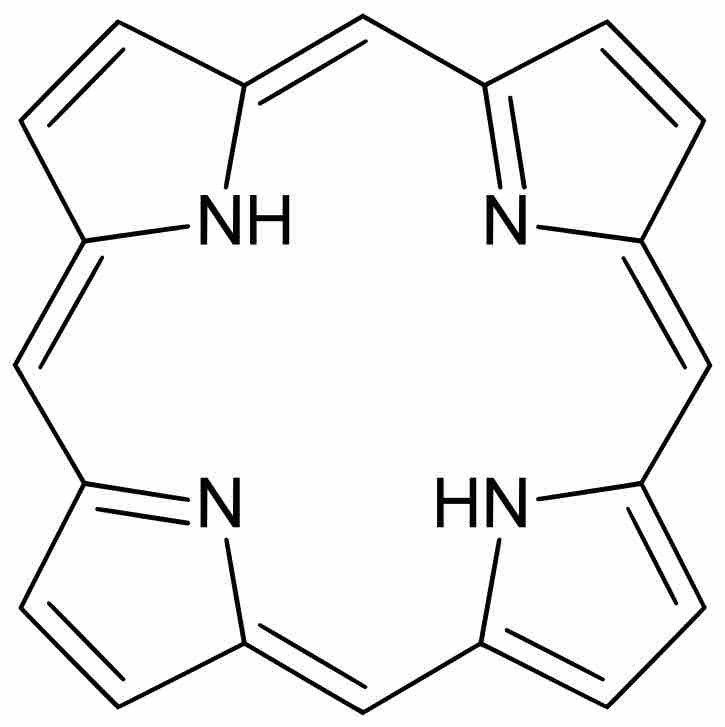
Structure of Porphine
Porphine is the simplest porphyrin, an aromatic organic compound.
Porphyrins are the conjugate acids of ligands that bind metals to form complexes. The metal ion usually has a charge of 2+ or 3+. A schematic equation for these syntheses is:
H2porphyrin + [MLn]2+ → M(porphyrinate)Ln-4 + 4 L + 2 H+
where M=metal ion and L=a ligand
A porphyrin without a metal ion in its cavity is a free base. Some iron-containing porphyrins are called hemes. Heme-containing proteins, or hemoproteins, are found extensively in nature. Hemoglobin and myoglobin are two O2-binding proteins that contain iron porphyrins. Various cytochromes are also hemoproteins. Several other heterocycles are related to porphyrins. These include corrins, chlorins, bacteriochlorophylls and corphins. Chlorins (2,3-dihydroporphyrin) are more reduced, contain more hydrogen than porphyrins, and feature a pyrroline subunit. This structure occurs in a chlorophyll molecule. Replacement of two of the four pyrrolic subunits with pyrrolinic subunits results in either a bacteriochlorin (as found in some photosynthetic bacteria) or an isobacteriochlorin, depending on the relative positions of the reduced rings. Some porphyrin derivatives follow Hückel's rule, but most do not.
The "committed step" for porphyrin biosynthesis is the formation of δ-aminolevulinic acid (δ-ALA, 5-ALA or dALA) by the reaction of the amino acid glycine with succinyl-CoA from the citric acid cycle. Two molecules of dALA combine to give porphobilinogen (PBG), which contains a pyrrole ring. Four PBGs are then combined through deamination into hydroxymethyl bilane (HMB), which is hydrolysed to form the circular tetrapyrrole uroporphyrinogen III. This molecule undergoes a number of further modifications. Intermediates are used in different species to form particular substances, but, in humans, the main end-product protoporphyrin IX is combined with iron to form heme. Bile pigments are the breakdown products of heme.