The Western blot (sometimes called the protein immunoblot) is a widely accepted analytical technique used to detect specific proteins in a given sample of tissue homogenate or extract. Western blot samples can be taken from whole tissue or from cell culture. Solid tissues are first broken down mechanically using either a blender (for larger sample volumes), a homogenizer (smaller volumes), or by sonication . Assorted detergents, salts, and buffers may be employed to encourage lysis of cells and to solubilize proteins. The technique uses gel electrophoresis to separate native proteins by 3-D structure or denatured proteins by the length of the polypeptide.
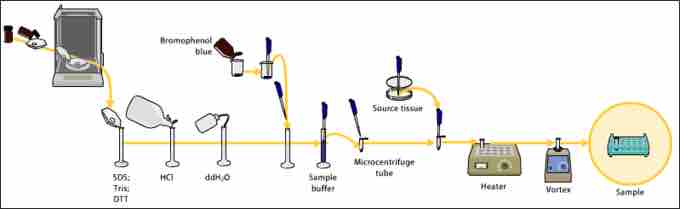
Western blot steps
Example preparation to use in the Western blot technique.
The proteins are then transferred to a membrane (typically nitrocellulose or PVDF), where they are stained with antibodies specific to the target protein. There are now many reagent companies that specialize in providing antibodies (both monoclonal and polyclonal antibodies) against tens of thousands of different proteins belonging to signaling pathways or cell surface receptor antigens, or other cellular or soluble components. Commercial antibodies can be expensive, although the unbound antibody can be reused between experiments. This method is used in the fields of molecular biology, biochemistry, immunogenetics and other molecular biology disciplines. Other related techniques include using antibodies to detect proteins in tissues and cells by immunostaining and enzyme-linked immunosorbent assay (ELISA). This method originated in the laboratory of George Stark at Stanford. The name Western blot was given to the technique by W. Neal Burnette and is a play on the name Southern blot, a technique for DNA detection developed earlier by Edwin Southern. Detection of RNA is termed Northern blot.