Enzyme-linked immunosorbent assay (ELISA) is a method of quantifying an antigen immobilized on a solid surface. ELISA uses a specific antibody with a covalently coupled enzyme. The amount of antibody that binds the antigen is proportional to the amount of antigen present, which is determined by spectrophotometrically measuring the conversion of a clear substance to a colored product by the coupled enzyme.
Several variations of ELISA, seen in , exist but the most commonly used method is the sandwich ELISA. The sandwich assay uses two different antibodies that are reactive with different epitopes on the antigen with a concentration that needs to be determined. A fixed quantity of one antibody is attached to a series of replicate solid supports, such as plastic microtiter multi-well plate. Test solutions containing antigen at an unknown concentration are added to the wells and allowed to bind. Unbound antigen is removed by washing, and a second antibody which is linked to an enzyme is allowed to bind. This second antibody-enzyme complex constitutes the indicator system of the test. The antigen serves as bridge, so the more antigen in the test solution, the more enzyme-linked antibody will bind . The test solution is used in parallel with a series of standard solutions with known concentrations of antigen that serve as control and reference. The results obtained from the standard solutions are used to construct a binding curve of the second antibody as a function of antigen concentration. The concentration of antigens can be inferred from absorbance readings of standard solutions.
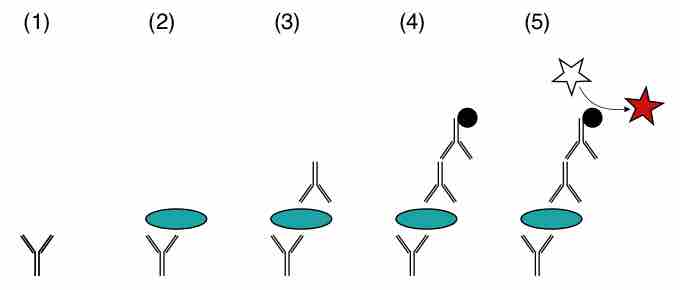
Sandwich ELISA
Two antibodies recognize different epitopes on same antigen.
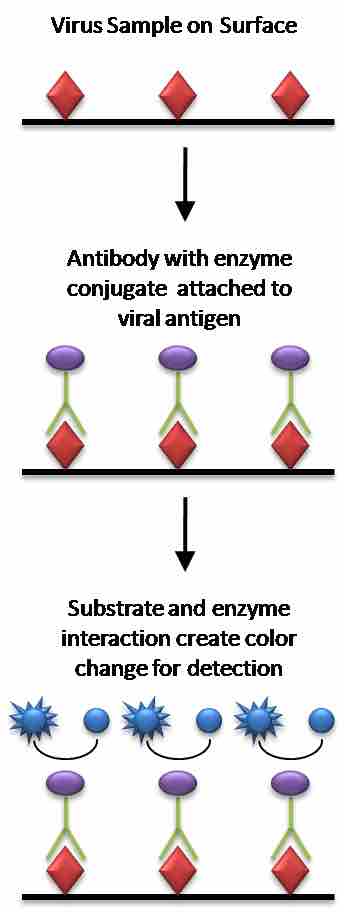
ELISA
Direct ELISA diagram using a viral antigen as an example.