Toll-like receptors (TLRs) are a class of proteins that play a key role in the innate immune system as well as the digestive system . They are single, membrane-spanning, non-catalytic receptors that recognize structurally conserved molecules derived from microbes. Once these microbes have breached physical barriers such as the skin or intestinal tract mucosa, they are recognized by TLRs, which activate immune cell responses.
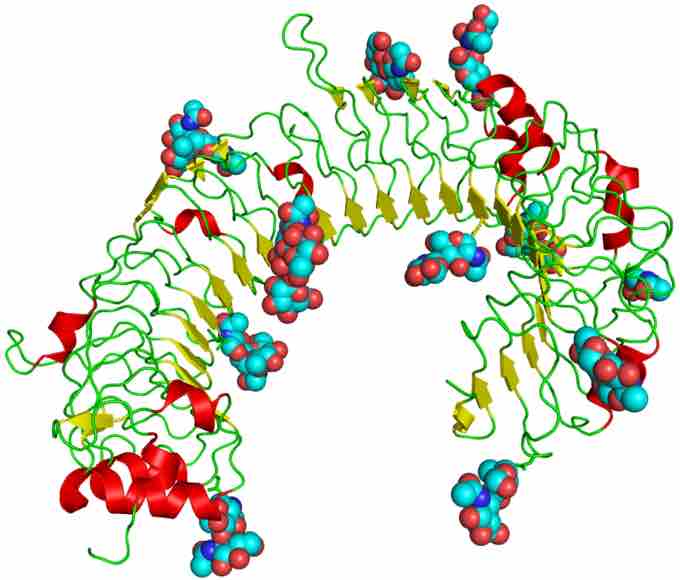
TLR3
The curved leucine-rich repeat region of Toll-like receptors, represented here by TLR3
TLRs are a type of pattern recognition receptor (PRR) and recognize molecules that are broadly shared by pathogens but distinguishable from host molecules, collectively referred to as pathogen-associated molecular patterns (PAMPs). TLRs together with the Interleukin-1 receptors form a receptor superfamily, known as the "Interleukin-1 Receptor/Toll-Like Receptor Superfamily"; all members of this family have in common a so-called TIR (Toll-IL-1 receptor) domain.
Because of the specificity of Toll-like receptors (and other innate immune receptors) they cannot easily be changed in the course of evolution, these receptors recognize molecules that are constantly associated with threats (i.e., pathogen or cell stress) and are highly specific to these threats (i.e., cannot be mistaken for self molecules). Pathogen-associated molecules that meet this requirement are usually critical to the pathogen's function and cannot be eliminated or changed through mutation; they are said to be evolutionarily conserved. Well-conserved features in pathogens include bacterial cell-surface lipopolysaccharides (LPS), lipoproteins, lipopeptides, and lipoarabinomannan; proteins such as flagellin from bacterial flagella; double-stranded RNA of viruses; or the unmethylated CpG islands of bacterial and viral DNA; and certain other RNA and DNA. For most of the TLRs, ligand recognition specificity has now been established by gene targeting (also known as "gene knockout"): a technique by which individual genes may be selectively deleted in mice. See the table below for a summary of known TLR ligands.
TLRs are believed to function as dimers. Though most TLRs appear to function as homodimers, TLR2 forms heterodimers with TLR1 or TLR6, each dimer having a different ligand specificity. TLRs may also depend on other co-receptors for full ligand sensitivity, such as in the case of TLR4's recognition of LPS, which requires MD-2. CD14 and LPS-Binding Protein (LBP) are known to facilitate the presentation of LPS to MD-2 .
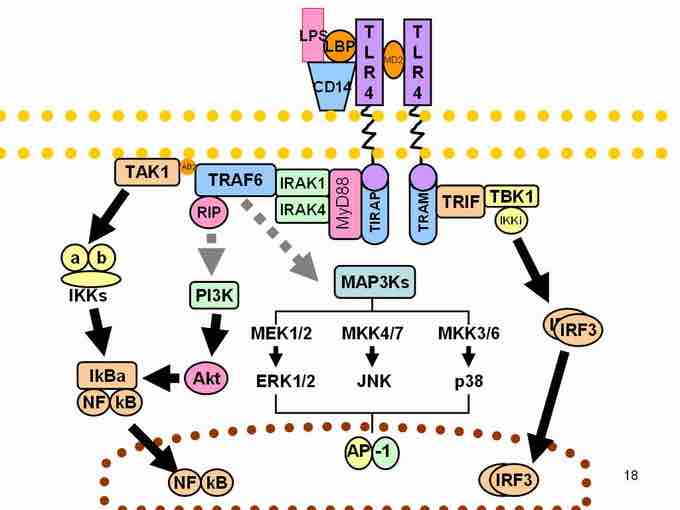
Signaling pathway
Signaling pathway of Toll-like receptors. Dashed grey lines represent unknown associations.
The adapter proteins and kinases that mediate TLR signaling have also been targeted. In addition, random germline mutagenesis with ENU has been used to decipher the TLR signaling pathways. When activated, TLRs recruit adapter molecules within the cytoplasm of cells in order to propagate a signal. Four adapter molecules are known to be involved in signaling. These proteins are known as MyD88, Tirap (also called Mal), Trif, and Tram.
TLR signaling is divided into two distinct signaling pathways, the MyD88-dependent and TRIF-dependent pathway. The MyD88-dependent response occurs on dimerization of the TLR receptor, and is utilized by every TLR except TLR3. Its primary effect is activation of NFκB. Ligand binding and conformational change that occurs in the receptor recruits the adaptor protein MyD88, a member of the TIR family. MyD88 then recruits IRAK 4, IRAK1 and IRAK2. IRAK kinases then phosphorylate and activate the protein TRAF6, which in turn polyubiquinates the protein TAK1, as well as itself in order to facilitate binding to IKKβ. On binding, TAK1 phosphorylates IKKβ, which then phosphorylates IκB causing its degradation and allowing NFκB to diffuse into the cell nucleus and activate transcription.
Both TRL3 and TRL4 utilize the TRIF-dependent pathway, which is triggered by dsRNA and LPS, respectively. For TRL3, dsRNA leads to activation of the receptor, recruiting the adaptor TRIF. TRIF activates the kinases TBK1 and RIP1, which creates a branch in the signaling pathway. The TRIF/TBK1 signaling complex phosphorylates IRF3 allowing its translocation into the nucleus and production of Type I interferons. Meanwhile, activation of RIP1 causes the polyubiquination and activation of TAK1 and NFκB transcription in the same manner as the MyD88-dependent pathway.
TLR signaling ultimately leads to the induction or suppression of genes that orchestrate the inflammatory response. In all, thousands of genes are activated by TLR signaling, and collectively, the TLRs constitute one of the most pleiotropic yet tightly regulated gateways for gene modulation.
Toll-like receptors bind and become activated by different ligands, which, in turn, are located on different types of organisms or structures. They also have different adapters to respond to activation and are located sometimes at the cell surface and sometimes to internal cell compartments.