A biofilm is an aggregate of microorganisms in which cells adhere to each other on a surface. These cells are frequently embedded within a self-produced matrix of extracellular polymeric substance (EPS). Biofilm EPS, also referred to as slime, is a polymeric conglomeration composed of extracellular DNA, proteins, and polysaccharides. Biofilms may form on living or non-living surfaces and can be prevalent in natural, industrial, and hospital settings.
The microbial cells growing in a biofilm are physiologically distinct from planktonic cells of the same organism, which, by contrast, are single cells that may float or swim in liquid. Microbes form a biofilm in response to many factors, including cellular recognition of specific or non-specific attachment sites on a surface, nutritional cues, or exposure of planktonic cells to sub-inhibitory concentrations of antibiotics. When a cell switches to the biofilm mode of growth, it undergoes a phenotypic shift in behavior in which large suites of genes are differentially regulated.
Formation of a biofilm begins with the attachment of free-floating microorganisms to a surface. These first colonists initially form a weak, reversible adhesion to the surface via van der Waals forces. If the colonists are not immediately separated from the surface, they can anchor themselves more permanently using cell adhesion structures such as pili. Some species are not able to attach to a surface on their own but are able to anchor themselves to the matrix or directly to earlier colonists. It is during this colonization that the cells are able to communicate via quorum sensing using such products as AHL. Once colonization has begun, the biofilm grows through a combination of cell division and recruitment. The final stage of biofilm formation is known as development; this is the stage in which the biofilm is established and may change only in shape and size. The development of a biofilm may allow for an aggregate cell colony (or colonies) to be antibiotic-resistant.
In sum, the five stages of biofilm development are as follows:
- Initial attachment
- Irreversible attachment
- Maturation I
- Maturation II
- Dispersion
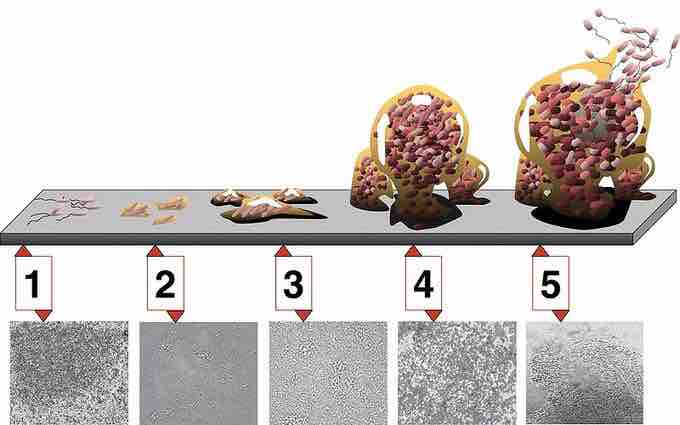
The Five Stages of Biofilm Development
Stage 1: initial attachment; stage 2: irreversible attachment; stage 3: maturation I; stage 4: maturation II; stage 5: dispersion. Each stage of development in the diagram is paired with a photomicrograph of a developing Pseudomonas aeruginosa biofilm. All photomicrographs are shown at the same scale.
Dispersal of cells from the biofilm colony is an essential stage of the biofilm life cycle. Dispersal enables biofilms to spread and colonize new surfaces. Enzymes that degrade the biofilm extracellular matrix, such as dispersin B and deoxyribonuclease, may play a role in biofilm dispersal. Biofilm matrix-degrading enzymes may be useful as anti-biofilm agents. Recent evidence has shown that one fatty acid messenger, cis-2-decenoic acid, is capable of inducing dispersion and inhibiting growth of biofilm colonies. Secreted by Pseudomonas aeruginosa, this compound induces cyclo heteromorphic cells in several species of bacteria and the yeast Candida albicans. Nitric oxide has also been shown to trigger the dispersal of biofilms of several bacteria species at sub-toxic concentrations, so it shows potential for use in the treatment of patients that suffer from chronic infections caused by biofilms.
Bacteria living in a biofilm usually have significantly different properties from free-floating bacteria of the same species, as the dense and protected environment of the film allows them to cooperate and interact in various ways. One benefit of this environment is increased resistance to detergents and antibiotics, as the dense extracellular matrix and the outer layer of cells protect the interior of the community. In some cases antibiotic resistance can be increased a thousandfold. Lateral gene transfer is also greatly facilitated in biofilms and leads to a more stable structure.
However, biofilms are not always less susceptible to antibiotics. For instance, the biofilm form of Pseudomonas aeruginosa has no greater resistance to antimicrobials than do stationary-phase planktonic cells, although when the biofilm is compared to logarithmic-phase planktonic cells, the biofilm does show greater resistance to antimicrobials. This resistance to antibiotics in both stationary phase cells and biofilms may be due to the presence of persister cells.