MreB is a protein found in bacteria that has been identified as a homologue of actin, as indicated by similarities in tertiary structure and conservation of active site peptide sequence. The conservation of protein structure suggests the common ancestry of the cytoskeletal elements formed by actin and MreB, found in prokaryotes. Indeed, recent studies have found that MreB proteins polymerize to form filaments that are similar to actin microfilaments.MreB controls the width of rod-shaped bacteria, such as Escherichia coli. A mutant E. coli that creates defective MreB proteins will be spherical instead of rod-like. Also, bacteria that are naturally spherical do not have the gene encoding MreB. Prokaryotes carrying the mreB gene can also be helical in shape. MreB has long been thought to form a helical filament underneath the cytoplasmic membrane. However, this model has been brought into question by three recent publications showing that filaments cannot be seen by electron cryotomography and that GFP-MreB can be seen as patches moving around the cell circumference. It has also been shown to interact with several proteins that are proven to be involved in length growth (for instance PBP2). Therefore, MreB probably directs the synthesis and insertion of new peptidoglycan building units into the existing peptidoglycan layer to allow length growth of the bacteria.
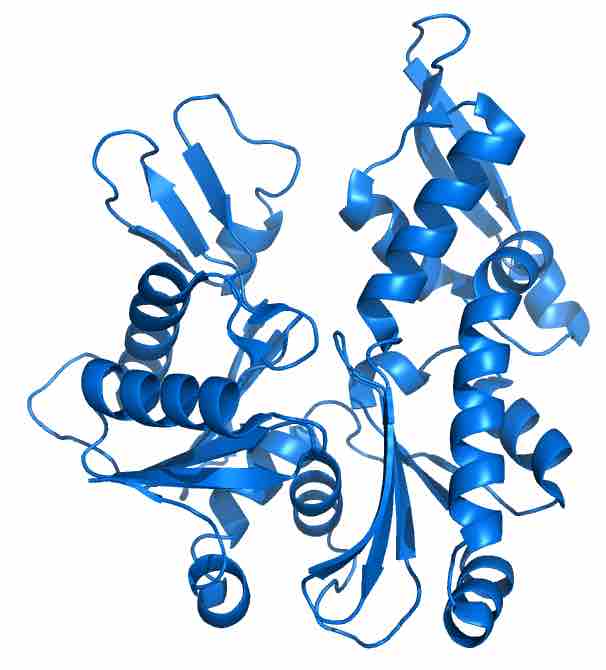
Atomic structure of MreB, a prokaryotic structural protein
Procaryotic MreB in cartoon representation. The fold of the protein is similar to its eukaryotic counterpart
MreB is a cytoskeleton element that assembles into filamentous structures within the bacterial cytoplasm. MreB and its homologs have been shown to interact and co-localize with cytoplasmic protein( MurB-G), membrane-imbedded proteins ( MreD, MraY and RodA), as well as other molecules with large periplasmic domain in organism. Recent research shows that peptidoglycan precursors are inserted into cell wall following helical pattern which is dependent on MreB, and it's reported that MreB also promote the GT activity of PBPs. This ability of MreB is because of RodZ, an inner membrane protein containing an 80-residue, N-terminal cytoplasmic region, and a 200-amino acid periplasmic C-terminal tail. RodZ co-localizes with MreB helices in a manner that is strictly dependent on its cytoplasmic region. MreB- RodZ complexes act as a major stabilizing factor in bacterial cell wall and ensure the insertion of new peptidoglycan in a spiral like fashion into the cell wall.