Membrane Permeability
As a phospholipid bilayer, the lipid portion of the outer membrane is impermeable to charged molecules. However, channels called porins are present in the outer membrane that allow for passive transport, across the outer membrane, of many ions, sugars, and amino acids. These molecules are present in the periplasm, the region between the cytoplasmic and outer membranes. The periplasm contains the peptidoglycan layer and also many proteins responsible for substrate binding or hydrolysis and the reception of extracellular signals. The periplasm is thought to exist as a gel-like state rather than a liquid due to the high concentration of proteins and peptidoglycan found within it. Because of its location between the cytoplasmic and outer membranes, signals received and substrates bound are available to be transported across the cytoplasmic membrane using transport and signalling proteins that are embedded there.
Antimicrobial Drugs
Examples of antimicrobial drugs that can target the microbial cell membrane to alter its functionality include polymyxin and gramicidin.
After binding to lipopolysaccharide (LPS) in the outer membrane of gram-negative bacteria, polymyxins disrupt both the outer and inner membranes . The hydrophobic tail is important in causing membrane damage, suggesting a detergent-like mode of action. Removal of the hydrophobic tail of polymyxin B yields polymyxin nonapeptide, which still binds to LPS but no longer kills the bacterial cell. However, it still increases the permeability of the bacterial cell wall to other antibiotics, indicating that it causes some degree of membrane disorganization. Gram-negative bacteria can develop resistance to polymyxins through various modifications of the LPS structure that inhibit the binding of polymyxins to LPS.
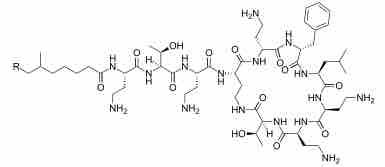
Polymyxin B
Polymyxin B has a hydrophobic tail that causes membrane damage.
Gramicidin is a heterogeneous mixture of six antibiotic compounds, gramicidins A, B, and C, making up 80%, 6%, and 14% respectively, all of which are obtained from the soil bacterial species Bacillus brevis and called collectively gramicidin D. Gramicidin D is made of linear pentadecapeptides, chains made of 15 amino acids. This is in contrast to gramicidin S, which is a cyclic peptide chain.
Gramicidin is active against gram-positive bacteria, except for gram-positive bacilli, and against selective gram-negative organisms, such as Neisseria bacteria. The therapeutic use of gramicidin is limited to topical application, as it induces hemolysis in lower concentrations and then bacteria cell death, so cannot be administered internally. Since the exterior epidermis is composed of dead cells, applying it to the skin's surface does not cause harm.
Gramicidin is used primarily as a topical antibiotic and is one of the three constituents of the consumer antibiotic polysporin ophthalmic solution. Gramicidin's bactericidal activity is a result of increasing the permeability of the bacterial cell membrane, allowing inorganic monovalent cations (e.g. Na+) to travel through unrestricted and thereby destroy the ion gradient between the cytoplasm and the extracellular environment.
Gramicidin D functioning as a channel was demonstrated by Hladky and Haydon, who investigated the unit conductance channel. In general, gramicidin channels are ideally selective for monovalent cations and the single-channel conductances for the alkali cations are ranked in the same order as the aqueous mobility of these ions. Divalent cations like Ca2+ block the channel by binding near its mouth so that it is essentially impermeable to divalent cations, and also excludes anions. Cl− in particular is excluded from the channel because its hydration shell is thermodynamically stronger than the shells of most monovalent cations. The channel is permeable to most monovalent cations, which move through the channel in single file. The channel is filled with about six water molecules, almost all of which must be displaced when an ion is transported. Thus, ions moving through the gramicidin pore carry a single file of water molecules. The flow of ion molecules and water molecules is known as flux coupling. In the presence of a second type of permeable ion, the two ions couple their flux as well. Like valinomycin and nonactin, the gramicidin channel is selective for potassium over sodium but only slightly so. It has a permeability ratio of 2.9. Though it is impermeable to anions, there are conditions under which some anion permeation may be observed. Its ability to bind and transport cations is due to the presence of cation-binding sites, one strong and the other weak, in the channel.