The cell wall is the principal stress-bearing and shape-maintaining element in bacteria. Its integrity is thus of critical importance to the viability of a particular cell. In both gram-positive and gram-negative bacteria, the scaffold of the cell wall consists of a cross-linked polymer peptidoglycan. The cell wall of gram-negative bacteria is thin (approximately only 10 nanometers in thickness), and is typically comprised of only two to five layers of peptidoglycan, depending on the growth stage. In gram-positive bacteria, the cell wall is much thicker (20 to 40 nanometers thick).
While the peptidoglycan provides the structural framework of the cell wall, teichoic acids, which make up roughly 50% of the cell wall material, are thought to control the overall surface charge of the wall. This affects murein hydrolase activity, resistance to antibacterial peptides, and adherence to surfaces. Although both of these molecules are polymerized on the surface of the cytoplasmic membrane, their precursors are assembled in the cytoplasm. Any event that interferes with the assembling of the peptidoglycan precursor, and the transport of that object across the cell membrane, where it will integrate into the cell wall, would compromise the integrity of the wall. Damage to the cell wall disturbs the state of cell electrolytes, which can activate death pathways (apoptosis or programmed cell death). Regulated cell death and lysis in bacteria plays an important role in certain developmental processes, such as competence and biofilm development. They also play an important role in the elimination of damaged cells, such as those irreversibly injured by environmental or antibiotic stress. An example of an antibiotic that interferes with bacterial cell wall synthesis is Penicillin. Penicillin acts by binding to transpeptidases and inhibiting the cross-linking of peptidoglycan subunits. A bacterial cell with a damaged cell wall cannot undergo binary fission and is thus certain to die .
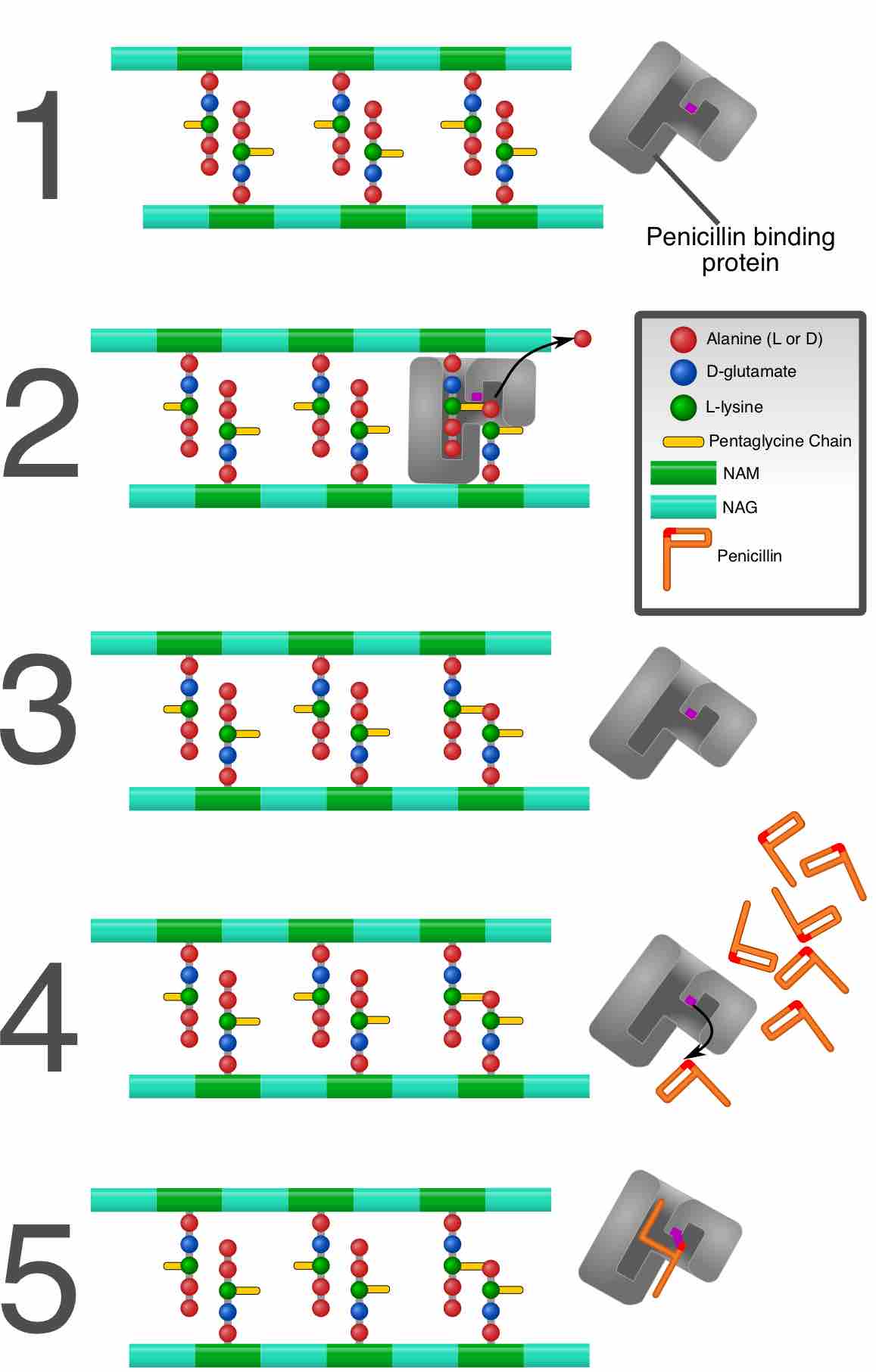
Penicillin mechanism of action
Penicillin acts by binding to penicillin binding proteins and inhibiting the cross-linking of peptidoglycan subunits.