Isomers in Coordination Compounds
As with other compounds, there are several kinds of coordination complex isomers. Stereoisomers occur when the ligands have the same bonds, but the bonds are in different orientations relative to one another. Structural isomerism occurs when the bonds are different. Isomers are distinct compounds that can have different physical properties such as color, crystal structure, and melting point.
Cis and Trans Stereoisomers
In octahedral complexes—with four of one ligand and two of another—and square planar complexes—with two of one ligand and two of another—there are two different arrangements of the same atoms with the same bonds. These different arrangements are called cis and trans. In cis molecules, the two ligands are on the same side of the complex. This means that in both octahedral and square planar complexes the bond angle between the two similar ligands will be 90 degrees. In trans molecules, the similar ligands are on the opposite sides of the molecules, meaning the bond angle is 180 degrees. Tetrahedral molecules do not show stereoisomerism.
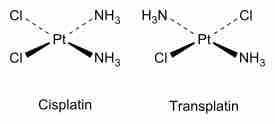
Cisplatin and transplatin
Cis and trans isomers for square planar complexes.
An example of cis and trans isomers can be seen for platin. Cisplatin is used as an anti-cancer drug because it can bind to the DNA of rapidly growing cells and prevent replication. The trans form of this chemical is not as effective at binding to the DNA.
Fac and Mer Stereoisomers
When three identical ligands occupy one face of an octahedron, the isomer is said to be facial, or fac. In a fac isomer, any two identical ligands are adjacent or cis to each other. If these three ligands and the metal ion are in one plane, the isomer is said to be meridional, or mer. A mer isomer can be considered as a combination of a trans and a cis, since it contains both trans and cis pairs of identical ligands.
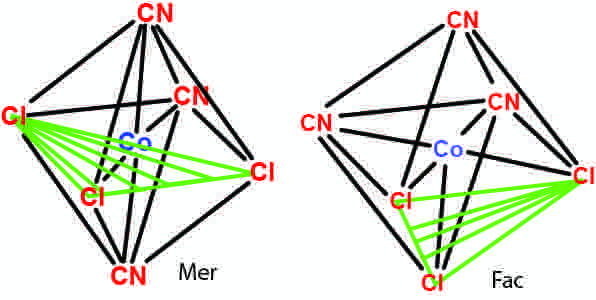
Fac and mer isomers
Examples of fac and mer isomers.
Optical Stereoisomers
Optical isomerism occurs when a molecule is not superimposable with its mirror image. If you shine light through pure solutions of each optical isomer they would rotate the plane of polarized light in opposite directions. The symbol Λ (lambda) is used as a prefix to describe the left-handed propeller twist formed by three bidentate ligands. The symbol Δ (delta) is used as a prefix for the right-handed propeller twist.
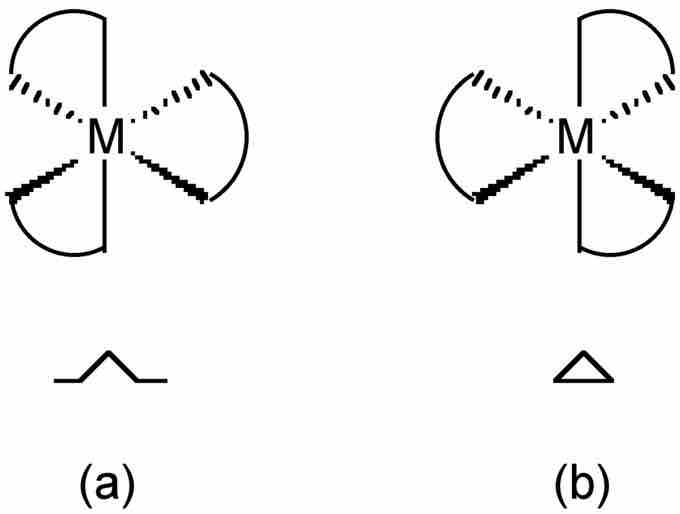
Optical isomers
The two optical isomers of a tris-chelate metal complex.
Structural Isomers
There are four types of structural isomerism:
- In ionisation isomerism, the isomers result in different ions in solution although they have the same composition. This type of isomerism occurs when the center ion of the complex is also a potential ligand. For example, pentaaminebromidocobalt(III) sulphate [Co(NH3)5Br]SO4 is violet and in solution gives a precipitate with barium chloride, confirming the presence of sulphate ion. Pentaaminesulphatecobalt(III) bromide [Co(NH3)5SO4]Br on the other hand, is red and tests negative for sulphate ion in solution. It gives a precipitate of silver bromide (AgBr) with silver nitrate (AgNO3).
- In solvate or hydrate isomerism, the isomers have the same composition but differ with respect to the number of solvent ligand molecules as well as the counter ion in the crystal lattice. For example [Cr(H2O)6]Cl3 is violet colored, [Cr(H2O)5Cl]Cl2·H2O is blue-green, and [Cr(H2O)4Cl2]Cl·2H2O is dark green.
- Linkage isomerism occurs with ambidentate ligands that can bind in more than one place. For example, NO2 can bind to a metal at either the N atom or an O atom.
- In coordination isomerism, both positive and negative ions of a salt are complex ions and the two isomers differ in the distribution of ligands between the cation and the anion. For example, [Co(NH3)6][Cr(CN)6] and [Cr(NH3)6][Co(CN)6].